| |
|
|
INTRODUCTION
The use of a medicinal product in accordance with the marketing
authorization, which defines the formulation, dosage, age, and
issued by the relevant regulatory body, is called the use of the
medicinal product in accordance with the on-label marketing
authorization. The purpose of authorizing a medicinal product is to
ensure that the medicinal product is tested for its efficacy, safety
and quality. When the drug is prescribed outside the examined
indications, the therapy may be less safe, effective and reliable,
because it is based exclusively on assumptions and extrapolation.
The justification for prescribing these drugs, especially in the
pediatric population, due to the large differences between children
and adults, even between children of different ages, in terms of
pharmacodynamic and pharmacokinetic responses to the drug, is being
examined.
Recently, the use of a drug that does not comply with the approved
guidelines related to the indication, age, dosage regime or route of
administration is becoming more common. Off-label use of drugs
includes the use of drugs in higher or lower doses, use for
indications not described in the summary of product characteristics,
use in children outside the range of years defined by the license,
use of alternative routes of administration and use of drugs in
indications when contraindicated for a given drug. The use of
off-label drugs is mainly related to prevention, diagnosis or
therapeutic measures that are in accordance with the relevant
legislation, with the primary goal of improving or improving the
health condition.
Off-label use of drugs should be distinguished from the use of drugs
without a license (off-license). Unlicensed use of drugs is
considered to be the use of a drug that is not registered in the
Republic of Serbia, but is in other countries, or that is
registered, but it should be translated into another formulation or
drug that is not registered (eg. for the treatment of rare
diseases). Unregistered medicines are medicines that have not been
approved by the regulatory body for marketing. Off-label use is
considered to be the use of a drug in a way different from the
manner described in the marketing authorization: use of the drug for
the treatment of an indication not listed in the summary of product
characteristics, use of the drug in the age group outside the
permitted range, use of the drug doses of the drug characteristics
listed in the summary.
The most common reasons for the use of unregistered drugs are
modifications of registered drugs (crushing the tablet to form a
suspension), drugs that are registered for use in adults, but the
formulation for use in pediatrics requires a special drug permit
(adult drug is used in minor doses for children), new drugs that
require special permission from the manufacturer (eg. caffeine
injection used in case of apnea due to lung immaturity). Use of
drugs outside the marketing authorization includes the use of drugs
in higher or lower doses, use for indications not described in the
summary of product characteristics, use in children outside the age
range defined by the license, use of alternative routes of
administration and use of drugs in indications when contraindicated
allow for a given drug.
Modern use of drugs in the treatment of diseases of children and
newborns is increasingly based on the use of off-label drugs due to
lack of adequate formulations for the pediatric population, lack of
appropriate therapeutic parallels for the treatment of children and
almost no clinical trials involving the pediatric population [1-4].
The thalidomide catastrophe (phocomelia in newborns) and the effect
of the use of chloramphenicol in children (gray baby syndrome)
initiated the process of testing and registration of drugs [5]. The
main goal of drug registration is to ensure that the drug is
quality, safe and effective. Unfortunately, large number of
medicines for children do not have a marketing authorization or
marketing authorization [6]. This suggests that for many drugs used
in children, evidence derived from pharmacokinetics, adequate
dosing, or formulation-related studies is lacking [7,8]. Focusing on
other factors influencing the pharmacokinetics and pharmacodynamics
of drug dosing has received little attention during drug development
in children. As a result, many drugs have been used outside of their
licensed recommendations, commonly known as off-label prescribing,
which has become an increasingly common prescribing trend in
children. Over-the-counter prescribing for children is widespread
mainly in systemically administered drugs, but also in locally
applied drugs [9].
Several factors leading to off-label prescribing in children have
been identified in the past. Subsequently, legislative, regulatory,
governmental, and professional initiatives were introduced and
implemented globally to obtain better data on the effects of drugs
on children and consequently to instruct health professionals to use
quality drugs that are effective for children and do not cause harm
when used. Initiatives to improve drug use in children were first
implemented in the United States from 1994 to early 2000. [10-13].
Almost a decade later, other countries (European Union, Canada,
Australia, Japan, China and Korea) as well as international
institutions (World Health Organization and the International
Council for the Harmonization of Technical Requirements for
Pharmaceutical Medicines for Human Use) have joined [14]. Data from
the literature show that most initiatives taken in the past have
been aimed at encouraging increased research on the use of drugs in
children, in order to improve the registration process and enable
the safe use of drugs in the pediatric population.
However, despite numerous global initiatives, the number of clinical
trials conducted in children is still insufficient, ie. the use of
drugs in children is rarely based on evidence from clinical trials
[15].
The aim of this study is to provide an overview of the global trend
and prevalence of prescribing off-label drugs from 1996 to 2016, and
to suggest future directions related to studies related to off-label
prescribing in children.
METHODOLOGY
Data collection was performed by electronic search of the PubMed
index database and Google Scholar. The literature search and
selection protocol has been defined using the PRIZMA method [16].
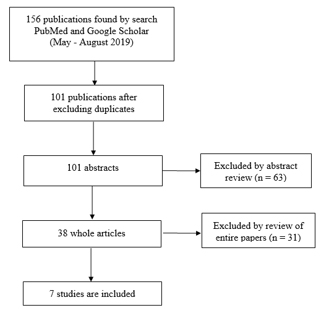
The corresponding flow diagram is graphically shown in Figure 1. The
search was performed in the period from May to August 2019. Selected
and presented in the paper are studies published in the period from
1996 to 2015. Searched keywords are: off label drug, pediatric
medicine, use in pediatrics. Original research was included which
provided data on the extent of use of off-label and unlicensed drugs
in the pediatric population as well as one systematic review.
Criteria for inclusion were: 1) published texts in full text in the
period from January 1996 to December 2016; 2) articles in Serbian
and English; 3) studies showing data on the results of the
prevalence of prescribing drugs outside the use permit for children;
4) off label use of cardiac, respiratory, antiallergic, oncological,
analgesic drugs and antibiotics
Exclusion criteria were: 1) notes and conferences; 2) off label use
of other therapeutic groups of drugs. The title and summary of the
articles have been carefully examined to determine the inclusion of
the study in this review. The following information was extracted
from the eligible studies: 1) study identification; 2) study details
(study design, setting, study period, method); 3) defining off-label
drug administration; 4) source references; 5) quantification of
outcomes; 6) results
Figure 1. PRIZMA diagram
RESULTS AND DISCUSSION
During the research, 101 studies were identified, of which 7 were
presented in this article, with the aim of presenting off-label use
of drugs in different therapeutic groups: cardiac, respiratory,
antiallergic drugs, antibiotics, oncology drugs and analgesics.
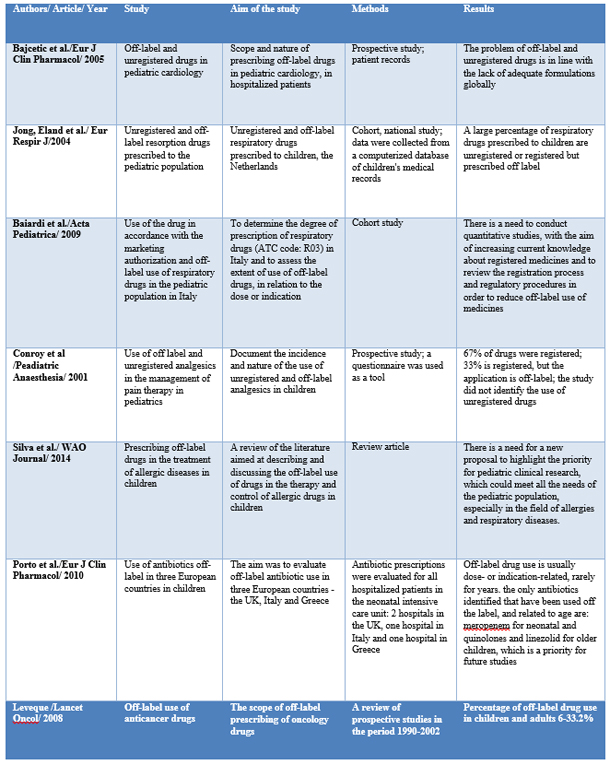
In a study conducted at the Department of Pediatric Cardiology, 544
patients participated in the University Children's Hospital in
Belgrade and included 2,037 prescriptions, with 102 different drugs,
of which 41% were registered drugs, 11% unregistered and 47%
prescribed drugs. off -label. Drugs are prescribed off-label: due to
age 21% and due to a different dose 26%. The largest number of
unregistered and off-label drugs (72%) is prescribed to children
aged between 2-11 years. Katopil is the only registered ACE
inhibitor for use in the pediatric population and is one of the most
prescribed drugs in this study, with one-third of prescriptions
being prescribed off-label in relation to the dose of katopil [17].
In a national cohort study conducted in Italy, in the period
2002-2006, medical records of children under 14 years of age were
analyzed, and the degree of prescribing drugs belonging to the ATC
code R03 - ß mimetics, inhaled glucocorticoids, inhaled
anticholinergics, combined formulations, antiallergic drugs,
xanthines and leukotriene receptor antagonists. 90% of R03
prescriptions included 11 active substances or combinations. Inhaled
glucocorticoids are the most prescribed off-label, with 19% in terms
of age and 56% in terms of indications for use. The largest number
of off-label drugs was in children younger than 2 years [18].
In the cohort study, conducted in the Netherlands, the largest
number of prescribed drugs - off-label and unregistered - was also
the largest in the group of children aged 1 month to 2 years. The
one-year cumulative risk of off-label and unregistered drugs is 45%,
among children with at least one prescription for a respiratory drug
[19].
In a prospective study, which lasted from February to March 2000, at
the Children's Clinic in Great Britain, in the intensive care and
acute care wards, analgesics used in children were classified into
those used in accordance with the marketing authorization and those
are applied off-label, in accordance with the valid drug registries
in the UK. The study included 715 prescriptions, of which 67% were
licensed drugs, prescribed in accordance with the summary of product
characteristics, and 33% were licensed drugs, but prescribed outside
the use permit. Diclofenac, pethidine and morphine are mostly
prescribed off-label, while drugs are most often prescribed
off-label, in terms of dose. The high percentage of off-label use of
this drug, shown in this study, is explained by the fact that
diclofenac is not approved for pain therapy in children, but that it
has been shown to be effective in adults intra- and postoperatively
[20].
The Morais-Almeida M study (2013) showed that the most prescribed
off-label drugs were nasal corticosteroids, 76% of the total number
of prescription drugs [21], while 22% were off-label antihistamines.
In other studies, off-label administration of antihistamines varied
between 4.5 -43%. Cetirizine, levocetirizine and loratadine have
been most studied in terms of long-term safety when used in the
pediatric population. Despite pharmacokinetic studies conducted for
next-generation antihistamines, long-term safety studies in children
are lacking [22].
A study conducted in three European countries, Italy, Great Britain
and Greece, evaluated the off-label use of antibiotics, as the most
frequently prescribed drugs for children. The number of prescribed
drugs with an unregistered dose was high in all three countries in
the neonatology departments, but the number was significantly higher
in Italy compared to the United Kingdom. Antibiotics that are most
often prescribed outside the recommended dose are aminoglycosides,
specifically amikacin and gentamicin. The most common clinical
indication for use outside the recommended range is suspected or
confirmed diagnosis of sepsis, although significant use of drugs
outside the recommended doses in medical prophylaxis was more common
in Italy and Greece, compared to the United Kingdom. The most
frequently prescribed antibiotics prescribed outside the registered
indication are fluoroquinolones in Great Britain and ampicillin and
gentamicin in Italy and Greece, while the most common indications
were suspected sepsis or diagnosed sepsis.
In the pediatric ward, antibiotics most commonly prescribed outside
the registered dose are amoxicillin clavulanate in Italy, cefuroxime
in Greece, and gentamicin in the United Kingdom. Doses were higher
than recommended in Italy and Greece and lower than recommended in
the UK. The most common dosages outside the registered
recommendations were indications - sepsis, lower respiratory tract
infections and surgical prophylaxis in all three countries,
regardless of prevalence. Off-label in terms of dose was most common
in the group of children aged 28 days - 23 months [23].
The use of anticancer drugs is precisely described in the drug
authorization in terms of the type or subtype of the tumor and the
length of treatment. Prescribing anticancer drugs is believed to be
often prescribed outside the use permit, while a small number of
studies have been conducted, in order to obtain a realistic state.
Prospective studies, conducted between 1990 and 2002, indicated a
proportion of off-label drug use in children and adults. Most
off-label drugs were for palliative care of patients, some were
associated with a better clinical effect and in the treatment of
specific tumors, they were part of standard therapy [24].
Table 1: Tabelar view of studies presented in this
article
CONCLUSION
According to the analysis of the literature, the prevalence of
prescribing off-label and unregistered drugs in the pediatric
population is evident and very widespread in the past in intensive
care units.
Medicines prescribed for children should be registered for use in
the pediatric population and used in accordance with approved
indications for children, whenever possible. Although there are
indications that the use of off-label and unregistered drugs has
more benefits than the risk that the use of that drug poses, this
leads to an increasing use of these drugs even when such use is not
justified, ie. it may be less effective or harmful.
The lack of indications for use in children, in relation to the dose
or inadequate formulation for the pediatric population may prevent
children from receiving effective therapy or may lead to errors in
the routes of administration of the drug.
The increase in the prevalence of off-label drug use suggests that
legislative, regulatory initiatives are not sufficient to improve
drug use in children. Aspects of behavior and knowledge related to
off-label prescribing as well as efforts to integrate evidence into
practice must also be assessed and consolidated as part of a joint
effort to reduce prescribing gaps for children.
It is necessary to take measures for a more rational use of
medicines in pediatrics, which include the collaboration of health
workers in order to provide medicines for children that are proven
to be effective, high quality and safe to use.
REFERENCES
- Goločorbin-Kon S. i dr. Lekovi u prometu 2014. Novi Sad:
OrtoMedics
- Bajčetić M, Uzelac Vidonja T. Raspoloživost, efikasnost i
kvalitet lekova u pedijatriji, Arhiv za farmaciju 2012, 62:
279-87.
- Krajnović D, Arsić J. Etička pitanja u pedijatrijskim
kliničkim studijama: izazovi i problemi kod pacijenata sa retkim
bolestima. JAHR 2014; 10: 277-89.
- Krajnović D. Etički i društveni aspekti u vezi sa retkim
bolestima. U: Drezgić R, Radinković Ž, Krstić P (ured.) Horizont
bioetike: moral u doba tehničke reprodukcije života, Beograd:
Univerzitet u Beogradu-Instistit za filozofiju i društvenu
teoriju 2012: 231-52.
- Mandić I, Krajnović D.Talidomidska tragedija - lekcija iz
prošlosti. Timočki medicinski glasnik 2009;34(2):126-34.
- Riedel C, Lehmann B, Broich K, Sudhop T. Improving drug
licensing for children and adolescents: position paper from the
More Medicines for Minors Symposion 8 June 2015 in Bonn.
Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz.
2016; 59:1587–92.
- Coté CJ, Kauffman RE, Troendle GJ, Lambert GH. Is
the“therapeutic orphan” about to be adopted? Pediatrics. 1996;
98:118–23.
- Rocchi F, Tomasi P. The development of medicines for
children. Part of a series on Pediatric Pharmacology, guest
edited by Gianvincenzo Zuccotti, Emilio Clementi, and Massimo
Molteni. Pharmacol Res. 2011; 64:169–75.
- Ufer M, Rane A, Karlsson Å, Kimland E, Bergman U. Widespread
off-label prescribing of topical but not systemic drugs for
350,000 paediatric outpatients in Stockholm. Eur J Clin
Pharmacol. 2003; 58:779–83.
- Nahata MC. New regulations for pediatric labeling of
prescription drugs. Ann Pharmacother. 1996; 30:1032–3.
- Suydam LA, Kubic MJ. FDA’s implementation of FDAMA: an
interim balance sheet. Food Drug Law J. 2001; 56:131–5.
- Ward RM, Kauffman R. Future of pediatric therapeutics:
reauthorization of BPCA and PREA. Clin Pharmacol Ther. 2007;
81:477–9.
- Fain K, Daubresse M, Alexander GC. The Food and Drug
Administration Amendments Act and postmarketing commitments.
JAMA. 2013; 310:202–4.
- Hoppu K, Anabwani G, Garcia-Bournissen F, Gazarian M, Kearns
GL, Nakamura H. et al. The status of paediatric medicines
initiatives around the world—what has happened and what has not?
Eur J Clin Pharmacol. 2012; 68:1–10.
- Corny J, Lebel D, Bailey B, Bussières JF. Unlicensed and
offlabel drug use in children before and after pediatric
governmental initiatives. J Pediatr Pharmacol Ther. 2015;
20:316–28.
- Dijkers, M., Introducing GRADE: a systematic approach to
rating evidence in systematic reviews and to guideline
development. KT Update (1)5. Austin, TX: SEDL, Center on
Knowledge Translation for Disability and Rehabilitation
Research, 2013. Available from:
http://www.ktdrr.org/products/update/v1n5/dijkers_grade_ktupdatev1n5.pdf
- Bajcetic M, Jelisavcic M, Mitrovic J, Divac N, Simeunovic S,
Samardzic R. et al. Off label and unlicensed drugs use in
paediatric cardiology, Eur J Clin Pharmacol 2005; 61: 775–9.
- Jong G.W. T, Eland I.A, Sturkenboom M.C.J.M,van den Anker
J.N, Stricker B.H.C. Unlicensed and off-label prescription of
respiratory drugs to children, Eur Respir J 2004; 23: 310–3.
- Baiardi1 P, Ceci1 A, Felisi M, Cantarutti L, Girotto S,
Sturkenboom M. et al. In-label and off-label use of respiratory
drugs in the Italian paediatric population, Acta Peadiatrica
2010; 99: 544–9 .
- Conroy S, Peden V. Unlicensed and off label analgesic use in
paediatric pain management, Paediatric Anaesthesia 2001, 11:
431-6.
- Morais-Almeida, M., & Cabral, A. J., Off-label prescribing
for allergic diseases in pre-school children. Allergologia et
Immunopathologia 2014; 42(4): 342-7.
doi:10.1016/j.aller.2013.02.011
- Silva D, Ansotegui I, Morais-Almeida M. Off-label
prescribing for allergic diseases in children, World Allergy
Organization Journal 2014; 7:4.
- Porta A, Esposito S, Menson E, Spyridis N, Tsolia M,
Sharland M. et al., Off-label antibiotic use in children in
three European countries, Eur J Clin Pharmacol 2010; 66:919–27.
- Dominique L, Off-label use of Anticancer Drugs, Lancet Oncol
2008; 9:1102-07.
|
|
|
|


