| |
|
|
INTRODUCTION
The clinical picture of myocarditis is diverse [1]. Myocarditis
(MY) can be the cause of sudden cardiac death in young adults
without known heart disease in 20%, idiopathic ventricular
tachycardia (VT) in 30%, acute heart failure in 10% [2,3]. MY is one
of the leading causes of sudden cardiac death and dilated
cardiomyopathy (DCM) in young people [4,5]. In the clinical series
of sudden cardiac death, MY is the third leading cause after
hypertrophic cardiomyopathy and congenital and atherosclerotic
coronary artery disease. [6]. Autopsy studies show that MY is a
common cause of DCM in biopsy-proven myocarditis but with large
variation from batch to batch: from 0.5% to 67%, the median is
10.3%. Due to the possibility of clinically silent disease and
infrequent myocardial biopsy, the exact frequency: incidence and
prevalence of MY and inflammatory cardiomyopathy (ICM) is unknown
[7,8]. Myocarditis (MY) or myocardial inflammation can be the result
of multiple causes, but is commonly associated with infectious
agents and more than 20 viruses that damage the myocardium by direct
invasion, production of cardiotoxic substances, and inflammation,
with or without persistent infection and autoimmune reactions to
cardiac epitopes [7,9,10,11]. AVMY is one of the biggest challenges
in terms of both diagnosis and therapy [7,12]. Clinical
classification of AVMY [7,13]:
- Possible subclinical acute myocarditis (typical viral
syndrome without cardiac symptoms and with ECG changes, positive
biomarkers of CK-MB and troponin, with echocardiographic
findings: decreased EF and regional anomalies of left
ventricular wall mobility and changes in myocardial texture)
- Probable clinical acute myocarditis (all previous +
symptoms: pain, shortness of breath, palpitations, etc.)
- Definitive myocarditis (confirmed pathohistological,
immunohistochemical and PCR viral genome via EMB)
This classification has not yet been revised by cardiomagnetic
resonance imaging (CMR), which would be necessary. The term ICM was
introduced in 1995 by the World Health Organization [14] and
involves myocarditis with systolic dysfunction and/or left
ventricular dilatation, but it does not describe the phenotype and
does not define the cause [15]. By their course, viral myocardites
are divided into subacute and chronic, they are often talked about
but rarely proven[15].
There is a change in the most common types of viral myocarditis,
previously Coxsackie B viruses and adenoviruses, and in the last two
decades Parvo B19, herpes virus type 6, hepatitis C virus, and now
less commonly Coxsackie B viruses, adenoviruses, Epstein-Barr virus
and Cytomegalovirus [7,11,12]. Myocarditis can also develop in
patients with HIV infection, hepatitis C or Lyme disease. [7,11,12].
Proven cases of myocarditis caused by the SARS CoV-2 virus have been
occurring since 2019 during the COVID 19 epidemic, but not enough is
known about it [16-20].
Most patients with acute viral myocarditis recover without sequelae,
but some patients progress to chronic inflammatory and dilated
cardiomyopathy, heart failure, and become candidates for heart
transplantation [1,5,12,13,15].
To this day, there has not existed the so-called non-invasive gold
standard for AVMY diagnosis due to the low specificity and
sensitivity of traditional diagnostic tests, but the development of
cardiomagnetic resonance imaging is promising [12,21,22].
Endomycardial biopsy with pathohistological examination and the
presence of viral genome is the most reliable method, if
representative myocardial samples are obtained [7,9] and it allows
the application of a therapeutic algorithm, but this invasive
diagnosis is mostly reserved for more severe and unclear cases of
inflammatory cardiomyopathies. Therefore, the clinical picture, ECG,
biomarkers and imaging methods, primarily in practice the easiest
echocardiography and increasingly magnetic resonance imaging, can,
in the form of a mosaic, complement the diagnosis of myocarditis
based on the clinical picture and various diagnostic categories with
an ESC score of 2 or more points [11,12].
The main symptoms of AVMY are common: fatigue, palpitations, chest
pain, shortness of breath on exertion; physical examination reveals
tachycardia, weakened first S1 tone and often S3 gallop rhythm and
de novo mesosystolic murmur [13,15,21]. The usual ECG nonspecific
finding in clinically suspected AVMY is most commonly sinus
tachycardia and various dysrhythmias: ventricular and
supraventricular extrasystoles, rarely ventricular tachycardia and
atrial fibrillation, and less frequently bradycardia and heart
blocks; ECG changes in the ST segment and T wave are quite specific
for myocardial lesions: transient changes in the ST segment and T
wave, depression or elevation of the ST segment, deep negative T
waves, block of the left branch of the His bundle and sometimes
images of myocardial infarction [13,15,21].
Elevated cardiac troponins are detected in the laboratory and there
are also newer markers. In children with fulminant myocarditis,
higher levels of creatinine, lactate and aspartate transaminase
(AST) are associated with increased hospital mortality [23].
Natriuretic peptide (NT-pro-BNP) is elevated in children with acute
ICM and generally declines rapidly in recovery of left ventricular
function [24]. In adults, higher concentrations of interleukin-10
are associated with an increased risk of death. Myocardial
antibodies (AHAs) have been reported to predict an increased risk of
death or the need for transplantation. [25]. Circulating viral
antibody titers do not correlate well with tissue viral genomes and
are rarely useful for diagnostic use in practice due to their low
sensitivity [11,12,26].
NON-INVASIVE IMAGING TECHNIQUES. The concept of imaging
has evolved from a monomodality to a multimodality imaging strategy
where each test adds information that increases the specificity of
the diagnostic marker for the diagnosis of myocarditis. Transthorax
Echocardiography (TTE) is the most available method at the patient's
bed, which can be used to suspect myocarditis. Echocardiographic
signs of clinically suspected AVMY are variable and heterogeneous:
most often left ventricular dysfunction with regional segmental
kinetic disorders, left ventricular dilatation or pericardial
effusion, rarely intracardiac thrombus, but the finding can be
normal, too [11,12,27]. When the echocardiographic window is
inadequate, an important step in diagnostics is transesophageal
echocardiography [28]. Imaging of deformation by speckle tracking
echocardiography (speckle tracking strain) usually shows a reduced
longitudinal pattern of myocardial deformation but it is also a
non-specific sign of myocardial disease.The advantage of the method
is that it can recognize early changes in myocardial function before
we see them using "ordinary" or conventional methods based on
measuring the ejection fraction of the left ventricle (EF)
[29,30,31,32,33]. Reduction of global longitudinal deformation (GLS)
has a diagnostic value and affects the prognosis of the disease in
inflammatory cardiomyopathy and heart failure. Cardiac magnetic
resonance imaging (CMR) is useful in diagnosing AVMY and for
monitoring disease progression, and the presence of late gadolinium
accumulation (LGE) is the best independent predictor of cardiac
mortality [21,34,35]. CMR shows a gadolinium binding in the medial
part of the left ventricular myocardium and subepicardially, which
is completely different from the findings in ischemic cardiomyopathy
[9,11,12,35].
Endomyocardial biopsy (EMB) with pathohistological
examination and the presence of viral genome by means of PCR and
immunohistochemical evidence of inflammation is the most reliable
method and allows the application of a therapeutic algorithm, but
this invasive diagnosis is mostly reserved for severe cases and
cardiomyopathies [7,9]. If myocardial samples are not
representative, false-negative EMB findings are possible. Yet most
authorities support the concept that EMB should be the gold standard
for the diagnosis of definitive myocarditis [7,9,11,12].
The basis of AVMY treatment is the treatment of heart failure and
arrhythmias. Specific treatment for fulminant and acute AVMY is
antiviral therapy and for post-viral chronic autoreactive
myocarditis the treatment is immunosuppressive therapy with
corticosteroids and cyclosporine [36] and more recently with CD3
muromonab [22].
RESEARCH OBJECTIVES: To analyze the type and significance
of echocardiographic parameters and characteristics in the diagnosis
of clinically suspected acute viral myocarditis in everyday
practice. To determine the role of antiviral antibody titer dynamics
for the diagnosis of clinically suspected acute viral myocarditis
and to compare viral serology in relation to echocardiographic
parameters of diastolic and systolic function of the left ventricle.
MATERIAL AND METHODS
A retrograde transverse study was performed in the ten-year
period from 2006 to 2015, where 126 consecutive patients clinically
suspected of acute viral myocarditis, were isolated from the
database of the Office of Internal medicine ‘’Dr. Bastać’’, having
been clinically, echocardiographically and serologically monitored
due to the dynamics of antibody titers to cardiotropic antibodies.
The examined group had an average age of 43.3 ± 8.9 years, body mass
index BMI 27.8 ± 5.9, dominated by female gender-78 (62%). Mean
values of systolic and diastolic pressure on arrival were -127 ±
14/78 ± 11 mmHg. The control group had comparable characteristics:
103 persons with average age 46 ± 12 years, body mass index BMI 29.3
± 6.4, 53 persons (52%) female. Mean systolic and diastolic pressure
on arrival were 136 ± 14/71 ± 11 mmHg.
Exclusion criteria: Absence of hypertension, known coronary
heart disease, valvular defects of other relevant diseases and with
low pre-test probability (PTP) <15% on ischemic heart disease.
Inclusion criteria: the criteria of Dennert et al. from 2007 were
used first [7] and later were re-evaluated through the criteria of
the Working Group on Myocardial and Pericardial Diseases of the
European Society of Cardiology from 2013 for clinically suspected
myocarditis [11]. 2 criteria at least were required: one at least
from the group of clinical presentations and one at least from the
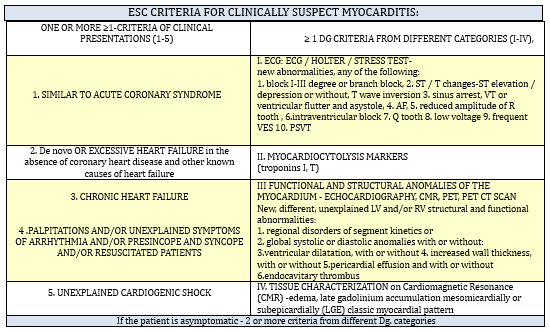
group of diagnostic categories as shown in the TABLE1 [11]
TABLE 1. The criteria of the Working Group on
Myocardial and Pericardial Diseases of the European Society of
Cardiology from 2013 for clinically suspected myocarditis [11]
METHODOLOGY. In addition to routine clinical methods:
anamnesis and physical examination, ECG, anthropometry, basic blood
biochemistry, echocardiography and serology of IgM and IgG antiviral
antibodies were performed on all of them. In individual cases,
radiography of the thorax was performed, as well as troponin T, pro
BNP and D dimer. Very rarely, the proposed examination on
cardio-magnetic resonance imaging was completed, while
endomyocardial biopsy was performed in only 2 patients.
Echocardiography. Echocardiographic examinations were performed
using Toshiba Power Vision 6000, Toshiba Xario CV and GE Vivid 7
multifrequency sector probes from 2.0 to 4.5 MHz with harmonic
imaging. All subjects underwent standard protocols, according to the
then valid recommendations [37,38]and they were interpreted in the
light of the latest recommendations for standards in performing
echocardiography [39,40]. Echocardiographic examinations were
performed by conventional M-mode and two-dimensional (B-mode)
echocardiography, and Doppler analysis of transmitral flow during
diastole was performed, as well as pulse tissue Doppler examination.
Of the structural parameters, left ventricular diameter (LA), left
ventricular telediastolic diameter (LVEDD), left ventricular
telesystolic diameter (LVESD), posterior left ventricular wall
thickness (PWTd), and interventricular septum IV were measured. The
criterion for left ventricular dilatation was the telediastolic
dimension of the left ventricle ≥54mm for women and ≥59mm for men)
[37]. Left ventricular volumes and left ventricular ejection
fractions (EF) were automatically calculated using the Teichholz
method and biplane Simpson method [37] and then the left ventricular
mass (LVM) was calculated by the Devereux formula and the left
ventricular mass index (LVMI).
( LVMI (g/m²) = [(TDD + ZZd + IVSd)3 –TDD3] x
1.05-13.4 / BSA(m²) [37]
Normal myocardial mass is up to 224 g for males and up to 162 g
for females. Myocardial mass index is less than 95 g/m² for females
and less than 115 g/m² for males. Diastolic function was assessed by
measuring the maximum velocity of the early (E) and late (A) phases
of ventricular filling, the deceleration time of the E velocity (DTE,
normally 160-200 ms), and by calculating the E/A ratio (normal E/A
≥0.8). Using the tissue Doppler technique, measurements of tissue
diastolic (e') and systolic velocities (S´) of the myocardium on the
septal and lateral sides of the mitral annulus were performed and
the mean value (e') was taken, and then the ratio E/e'was calculated
[38], as a surrogate for left ventricular filling pressure.
Diastolic function is categorized as:
(a) normal (E/A ≥0.8 - <1.5, E-DTE deceleration time> 160 ms, mean
E/e’≤8);
(b) Grade 1, impaired relaxation (E/A <0.8, DTE> 200 ms, mean
E/e’≤8);
(c) Grade 2, Pseudonormalization (E A ≥0.8 and <1.5, DTE 160–200 ms,
mean E/e’= 9–12);
(d) Grade 3, Restrictive pattern (E/A ≥1.5, DTE <160 ms, mean E
e’≥13).
Regional disorders in left ventricular contractility are segmental
hypokinesia, akinesia, dyskinesia. Changes in myocardial texture;
hyperechoic extensive subendocardial or transmural zones are a clear
finding while oval hyperechoic zones of the myocardium- most often
in the intraventricular septum are a controversial parameter. Only
extensive zones or 3 smaller zones with a diameter of ≥3 mm or
transmural involvement (signs of fibrosis and cicatrix) with
hypokinesia are significant. Based on the above criteria, clinically
suspected myocarditis was established - until 2015, these patients
were routinely tested for serum IgM and IgG antibodies to Parvo B19,
Coxsackie and Adenovirus, and exceptionally to less potential agents
(Ebstein Bar virus, cytomegalovirus, influenza virus, hepatitis C)
it was determined from 2 samples of paired sera at 2 to 8 weeks.
Antiviral antibodies and anti-heart antibodies were determined by
enzyme-linked immunosorbent assay (ELISA). Based on the
positivity of IgM antiviral antibodies, the examined group (A) was
divided into subgroups: A1 with elevated IgM antibody titer in
43 (32%) subjects (SUBGROUP A1) and A2 without elevated IgM antibody
titer (Group A2) - 83(68%) patients (SUBGROUP A2). Statistical
processing was done in the EXCEL database using the methods of
descriptive statistics, Student's-T test and Chi2 test.
RESULTS
126 patients (GROUP A) had clinically suspected myocarditis (KSVMy
with ≥2 ESC criteria). The most common symptoms were palpitations
107/126 (85%), chest pain 83/126 (66%) and fatigue, feeling tired,
shortness of breath and dyspnea on exertion 62/126 (49%) in various
combinations (TABLE 2)
TABLE 2. Symptoms, physical signs, and ECG changes
in 126 patients with suspected myocarditis and/or inflammatory
cardiomyopathy
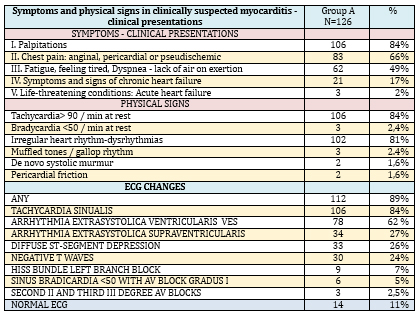
The physical finding in KSVMy (TABLE 2) was dominated by
tachycardia 106/126 (84%), irregular heart rhythm 102/126 (81%) and
much less frequent were more severe clinical forms: signs of cardiac
decompensation 21/126 (17%), (late inspiratory crackles in the
lungs, tachypnea, dyspnea at rest, swollen veins in the neck, late
inspiratory cracklesin the lungs, hepatomegaly, peripheral edema).
Objective, physical findings were normal in 14/126 (11%) subjects
Of the 126 cases of clinically suspected myocarditis, most had some
ECG changes-112/126 (89%), and with a normal ECG there were only
14/126 (11%) but echocardiographic changes were found in them. ECG
analysis (TABLE 2) registers a high frequency of nonspecific
disorders-dysrhythmias: sinus tachycardia in 112/126 (89%),
ventricular extrasystoles 78/126 (62%), supraventricular
extrasystoles 24/126 (19%) and electropathological changes for
clinically suspected myocarditis: diffuse ST segment depression
33/126 (26%), diffuse negative T waves 30/126 (24%) and His bundle
left branch block in 9 (7%) patients.
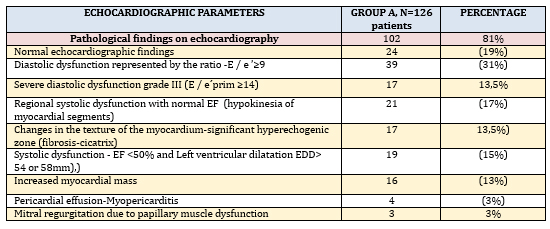
The analysis of parameters measured by transthoracic
echocardiography (TTE), in the presence of echocardiographic
criteria for KSVMy (TABLE 3) was dominated by left ventricular
diastolic dysfunction in 39/126 (31%), of which 17 (14%) had severe
diastolic dysfunction grade III.
Global left ventricular systolic dysfunction quantified by left
ventricular ejection fraction (EF) less than 50% (EF <50%) was found
in 19/126 (15%) and all had mild to moderate left ventricular
dilatation and criteria for inflammatory cardiomyopathy (ICM).
Increased left ventricular myocardial mass and left ventricular
myocardial mass index (LVMI) due to possible myocardial edema were
registered in 16 (13%) of these 19 patients. Regional systolic
dysfunction (hypokinesia of 2 or more left ventricular myocardial
segments), which, most commonly by distribution are not coronary
artery perfusion, was found in 21/126 (17%), with cicatrix present
in 11 patients, most commonly infero-postero-lateral.Myocardial
akinesia was not present in the study group and septal dyskinesia
was present in the left branch block (not taken into account) in 9
patients (7%). Changes in the texture of the myocardium - extensive
hyperechoic zone of the myocardium and fibrosis-cicatrix were found
in 17 (13%) subjects. However, 24/126 (19%) patients had a
completely normal echocardiographic finding, but with clinical and
ECG criteria for myocarditis.
TABLE 3. ECHOCARDIOGRAPHIC PARAMETERS IN
INDIVIDUAL DISTRIBUTION in clinically suspected myocarditis
In patients with clinically suspected myocarditis, the clinical
probability of viral etiology was diagnostically supported by
elevated IgM antibody titer in 43 (32%) subjects- (subgroup A1)
(CHART 1) while most were without elevated IgM antibody titer (Group
A2) - 83 (68%) patients.
CHART 1. Distribution of IgM serological
positivity in 43 (34%) of 126 patients examined for suspected recent
virus infection and evidence of autoimmune response via elevated AHA
antibodies serum titer
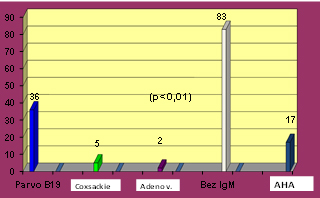
There is a predominance of IgM antibodies to Parvo B 19 virus in
36/43 (84%) patients (p <0.01) and only in 5/43 (12%) cases to
Coxsackie B and in 2/43 (5%) patients to Adenovirus. The majority of
patients were without elevated IgM antibody titer - subgroup A2 of
83 (68%) patients and about half of them -41/126 (32%) have only
elevated serum titer of IgG antibodies to cardiotropic which has no
diagnostic significance on its own, without IgM antibody titer
dynamics.
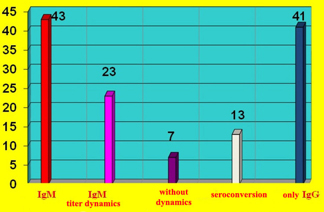
Clear IgM titer dynamics was recorded in 23/126 (18%) subjects and a
decrease in titer, with an increase in IgG titer (seroconversion) in
13/126 (10%) patients, while there were 7 patients without captured
titer dynamics (CHART 2)
CHART 2. Dynamics of IgM antibody titer to
cardiotropic viruses and IgM seroconversion to IgG in 43 patients
and increased only IgG antibodies in 41 patients without diagnostic
significance
Elevated IgG antibody titer has no diagnostic significance on its
own, without IgM antibody titer dynamics. In group A2 without IgaM,
41/126 (32%) had elevated serum IgG antibodies to cardiotropic
viruses, most often to parvo B19, adenovirus and coxsackie B. As
many as 42/126 patients (33%) did not have elevated IgM or IgG
titers. antiviral antibodies, but had clear criteria (2 and more)
for clinical myocarditis and 8 of them had elevated anti-heart
antibodies and signs of inflammatory CMP. Determination of
anti-heart autoantibodies (aha) was performed more recently in
severe cases of 17 inflammatory cardiomyopathy (CHART 1) of which 8
had antimyocardial autoantibodies, but their role has not yet been
defined.
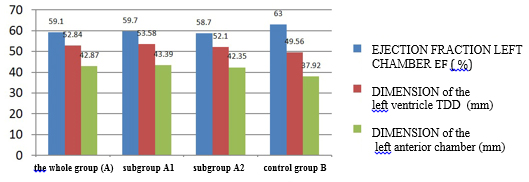
Quantitative echocardiographic parameters in patients with
clinically suspected myocarditis are shown in TABLE 4 and CHARTS 3
and 4.
TABLE 4. Quantitative echocardiographic parameters
in relation to viral serology in clinically suspected myocarditis
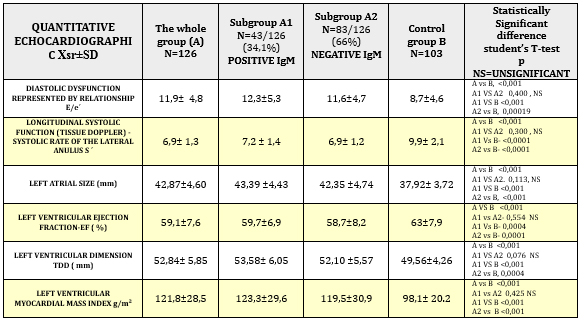
CHART 3. Quantitative echocardiographic parameters
of tissue Doppler: diastolic function and longitudinal systolic
function in relation to viral serology in clinically suspected
myocarditis
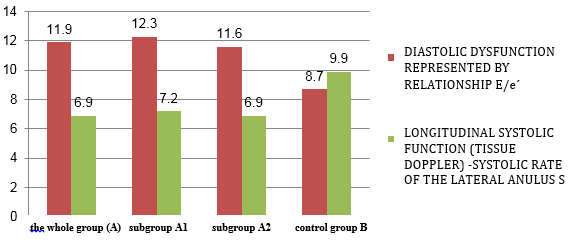
CHART 4. Echocardiographic parameters of left ventricular and atrial
systolic function and dimensions in relation to viral serology in
clinically suspected myocarditis
The whole group A of clinically suspected myocarditis compared to
control group B had statistically highly significantly reduced
parameters of systolic function (EF = 59.1 ± 7.6% vs. 63 ± 7.9%; p
<0.001) (Table 4 and Chart 3) including longitudinal systolic
function S´ via tissue Doppler 6.9 ± 1.3 cm / s vs. 9.9 ± 2.1; p
<0.001 (Table 4 and Chart 4).
Diastolic dysfunction (E/e´11.9 ± 4.8 vs. 8.7 ± 4.6; p <0.001) shown
in Table 4 and Graph 3, was highly significant in the study group
vs. control group. The increase in left ventricular telediastolic
dimension (TDD, EDD), myocardial mass index (LVMI) and left atrial
size (TABLE 4 and CHART 4) was statistically significantly increased
in the group of clinically suspected myocarditis. The whole group A
of clinically suspected myocarditis has a myocardial mass index
statistically significantly higher, which is explained by myocardial
edema and not hypertrophy as in hypertension.
Comparison of subgroups A1 and A2 did not find a statistically
significant difference between IgM positive and IgM negative
patients in relation to quantitative echocardiographic changes
(TABLE 4 AND CHARTS 3 AND 4), which means that elevated IgM antibody
titer and seroconversion do not indicate the degree of myocardial
damage and thus to a more severe form of myocarditis.
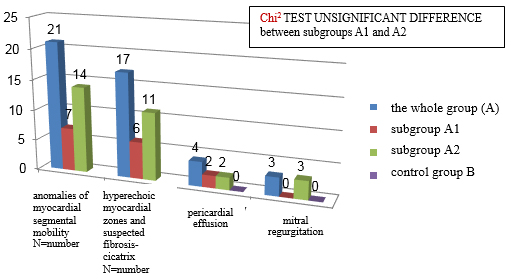
Qualitative echocardiographic changes are shown in CHART 5. These
changes do not occur in the control group, which indicates their
good specificity. As for quantitative echocardiographic parameters,
there is no statistically significant difference between subgroups
A1 and A2 (Xi2 test of insignificant difference).
CHART 5. Qualitative echocardiographic changes in
clinically suspected mycarditis
DISCUSSION
To this day, there has not existed the so-called gold standard
for the diagnosis of acute myocarditis due to the low specificity
and sensitivity of traditional diagnostic tests. Endomycardial
biopsy with pathohistological examination, immunohistochemistry and
the presence of viral genome is the most reliable method and allows
the application of a therapeutic algorithm, but this invasive
diagnosis is mainly reserved for more severe and unclear cases of
dilated and / or inflammatory cardiomyopathies. Acute viral
myocarditis is generally a mild and self-limiting consequence of
systemic infection with cardiotropic viruses [41]. However, patients
may develop temporary or permanent impairment of cardiac function,
including acute cardiomyopathy with hemodynamic compromise or severe
arrhythmia. Acute fulminate myocarditis is rare, it occurs primarily
in children as cardiogenic shock or pulmonary edema, and recognizing
it in time saves lives. EF usually returns almost to normal but
residual diastolic dysfunction may limit greater exertion in some
who have experienced fulminant myocarditis [13]. The proportion of
dilated cardiomyopathies (DCM) due to viral infection remains
controversial [42]. In the largest series of 1426 children,
myocarditis was the cause of DCM in 34% [43]. Accurate prediction of
CV risk in the earlier stages of myocarditis is especially important
due to the timely identification of high-risk patients [15].
The largest number of published studies rarely involve both initial
and follow-up biopsies [44,45,46] and have only outlined the initial
finding of EMB at the onset of symptoms. EMB-free series have been
diagnosed with chronic myocarditis based on clinical presentation,
elevated inflammatory markers, and image characterization in
patients with normal coronary angiography [47]. Previous studies
have estimated that 30% of DCM develops from myocarditis
[45,46,48,49].
Patients with acute myocarditis usually present chest pain, dyspnea,
or both, with tachycardia and dysrhythmias. [1,13,50,51,52]. In a
recent series of 245 patients with clinically suspected myocarditis,
the most common symptoms were fatigue (82%), exercise dyspnea (81%),
arrhythmias (55%, supraventricular and ventricular), palpitations
(49%), and chest pain at resting (26%) [53]. This is consistent with
our results, where arrhythmias and palitations dominated in 84%,
while chest pain was twice as common (66%). Viral prodrome of fever,
myalgia and respiratory symptoms occurs in between 20% and 80% of
cases, the patient can easily fail to report prodromes, so one
cannot rely on that in the diagnosis.
Of our 126 cases of clinically suspected myocarditis, most had some
electropathological ECG changes-112/126 (89%), and with a normal ECG
there were 14/126 (11%) so it cannot be used to rule out myocarditis.
However, in these 14 patients there were echocardiographic changes
and criteria for clinical presentation. Dysrhythmias have no
specificity for myocarditis, while ECG signs of myocardial damage,
depression or ST elevation, block of the left branch of the His
bundle speak in favour of myocardial lesions, but do not indicate
the cause. Estimation of ECG sensitivity for myocarditis is at about
47%, while the specificity is very low [52].Troponin, for example,
has an even lower sensitivity for myocarditis of 34% but a good
specificity of over 89% [52].
The analysis of parameters measured by transthoracic
echocardiography in the criteria for clinically suspected
myocarditis was dominated by left ventricular diastolic dysfunction,
represented by the ratio E / e´prim≥9 in 39/126 (31%), of which 17
(14%) patients, about half had severe diastolic dysfunction grade
III (E / e´prim ≥14). In one series of 147 patients with severely
reduced EF (23 ± 8%), 42% had diastolic dysfunction, but these were
more severe patients with inflammatory cardiomyopathy. Improvement
of diastolic function in 58% of these patients after treatment and
follow-up for about 6 months is prognostically important, as is
improvement in EF and it carries increasing prognostic value for
risk stratification [54]. Global left ventricular systolic
dysfunction (EF <50%) was found in only 19/126 (15%) of our patients
and all had mild left ventricular dilatation and criteria for
inflammatory cardiomyopathy. There was a significantly higher number
of patients with systolic dysfunction in the Italian study with
biopsy-proven myocarditis in a series of 41 pts [55], where left
ventricular systolic dysfunction was present in 69% and regional
contractility disorders in 64%, left ventricular hypertrophy due to
myocardial edema in 20%, changes in myocardial texture 23%,
ventricular thrombus in 15%, and restrictive left ventricular
filling pattern in 7%. Most of our patients had a normal ejection
fraction of 107 pts or 85%, which is an important prognostic factor
in most relevant studies [56,57,58]. In the registry of one German
centre on 210 EMB-proven myocarditis 50% or three times as many than
in our results had a reduced ejection fraction, due to the clinical
spectrum of severe patients with myocarditis who are sent for EMB.
After two years of follow-up and treatment with standard therapy for
heart failure, 26% normalized EF and 27% remained with decreased EF
[59]. Study by Grün S et al. [56] with a series of 222 consecutive
pts with EMB-proven viral myocarditis, gives the mortality rate of
19% with a median of 4.7 years. In general, about 1/4 of patients
with EMB-proven viral myocarditis go towards worsening cardiac
function and undergo or have a heart transplant or exit. [15].
Outcome predictors vary in various studies with EMB: NYHA class III
to IV persistence, left atrial dilatation, and EF improvement within
6 months are independent predictors of long-term outcome [42].
Kinderman I et al. state that high NYHA class, immune signs of
inflammation, and lack of beta-blockers in therapy are predictors of
poor outcome rather than histological characteristics of the Dallas
criteria or the presence of a viral genome [10].
Regional systolic dysfunction according to our research was
determined in 21/126 (17%) and in these cases cicatrix must be
excluded after asymptomatic infarction by stress echocardiographic
test by pharmacological or physical load and in inconclusive cases
by MSCT or invasive coronary angiography [60].
Echocardiography is an excellent tool for diagnosing and monitoring
patients with myocarditis and DCM. Speckle tracking echocardiography
(image of myocardial deformity) is of increasing importance in the
early stages of myocarditis and detection of progression to
cardiomyopathy [50].
The change in the type of myocarditis-causing virus is in line with
other studies [7,8,11,12], while one of the few recent studies from
Bulgaria finds the serological dominance of Coxsackie virus as a
possible cause of myocarditis [61]. Clear dynamics of IgM titer was
observed in a small number of patients in 23/126 (18%) persons with
Parvo B19 antibody dominance and a decrease in titer with an
increase in IgG titer (seroconversion) in 13/126 (10%) patients.
Increasing the titer dynamics of circulating antiviral antibodies
from acute to subacute and chronic phases may aid Dg viral
myocarditis with possible spontaneous recovery [13]. The sensitivity
of antiviral antibodies is low and estimated based on several
studies at 25-32% and specificity at 40% [52]. This tells of the
active process of infection anywhere in the body and contributes to
a possible causal diagnosis only with strong evidence of myocardial
involvement through valid ESC criteria for clinically suspected
myocarditis. In the most significant study on this topic, Mahfoud F.
et al [26] examined the serology of the virus and compared it with
PCR findings by endomicardial biopsy with histological and
immunohistochemical findings in 124 patients aged 40 ± 15 years with
suspected myocarditis. The viral genome was detected in the
myocardium by a polymerase chain reaction. Acute viral infection
with cardiotropic viruses was diagnosed by IgM in the initial sample
or IgG seroconversion in the next sample. Immunohistochemical signs
of inflammation were present in 54 patients. The viral genome was
detected in the myocardium of 58 patients (47%). In 20 patients
(16%), acute viral infection was diagnosed by serology, which is in
line with our result of 18%. But only 5 of 124 patients (4%) had
serological evidence of infection with the same virus detected by
EMB. The sensitivity of virus serology was only 9% and the
specificity was 77%. The lack of correlation between serology and
EMB is evidence against the routine use of viral serology in all
patients with clinically suspected myocarditis. The sensitivity of
viral serology is very low in relation to ECG and echocardiography,
and the specificity is moderate, and it should not be used routinely
in the evaluation of myocarditis, but in selected cases with ESC
criteria where CMR and EMB are not performed. It is known from
clinical experience that it is difficult to reassure some patients
of not having the "Coxsackie virus in their heart". The mental
burden of patients and attachment to "Coxsackie disease", which they
are convinced to carry for many years only on the basis of increased
serum IgG antiviral antibodies, is counterproductive from the
social-medical point of view. Anti-heart antibodies (AHA) do not
have an established role, because they occur in other diseases (CAD,
genetic CMP) and the sensitivity is similar to viral serology 25-30%
and specificity about 40% [52]. However, the pathohistological
Dallas criteria itself [52] without immunohistology and PCR have low
sensitivity 35 to 50% and good specificity 78 to 89%. Complemented
by immunohistochemistry and PCR identification of the virus genome,
the sensitivity is satisfactory 65% to 70% and the specificity
80-100%. Unfortunately even EMB has false negative findings,
depending on where the samples were taken and whether technically
enough tissue was taken.
A comparison between group A1 and group A2 did not reveal a
statistically significant difference in echocardiographic
parameters, which means that IgM antibodies and seroconversion do
not indicate more severe forms of myocarditis. There have been no
studies on this aspect so far.
The whole group A of clinically suspected myocarditis in relation to
the control group B has statistically highly significantly reduced
parameters of global systolic (EF = 59.1 ± 7.6 vs. 63 ± 7.9; p
<0.001) and longitudinal systolic function (S´ = 6.9 ± 1.3 vs 9.9 ±
2.1) which suggests that these subtle changes may lead us to think
of myocarditis in everyday clinical practice. In individual
distribution, systolic dysfunction is by half less represented than
diastolic (15% Vs 31%). Diastolic dysfunction, despite the
complexity of the assessment, is even more markedly reduced compared
to control group B, when we look at the most representative
parameter E/e´ (E/e´11.9 ± 4.8 vs. 8.7 ± 4.6; p <0.001). Dilatation
of the left atrium and left ventricle are highly significantly
increased mean values compared to the control group. Myocardial mass
and myocardial mass index are possible measures of myocardial edema
in myocarditis and are of significantly higher mean values in the
examined group vs. control group (121.8 ± 28.5 g/m² vs. 98.1 ± 20.2,
p <0.001) which is important for making a working diagnosis of
clinically suspected myocarditis, monitoring the course of the
disease and the effect of treatment. All echocardiographic changes
are without pathognomonicity and specificity for myocarditis, but
they have good diagnostic sensitivity. The ability of
echocardiographic parameters to predict the development of manifest
heart failure mortality and adverse CV events in the population of
inflammatory cardiomyopathy has been proven in a small number of
studies. In patients with clinically suspected myocarditis who have
not yet started treatment for heart failure and / or arrhythmias,
the association of both ejection fractions and diastolic dysfunction
with CV mortality has been confirmed [62,63,64]. Paradoxically in a
recent meta-analysis of Chen WH. and associates the presence of the
viral genome does not worsen the long-term prognosis of patients
with myocarditis or inflammatory cardiomyopathy. However,
virus-positive patients who have not received specific antiviral
treatment have a worse prognosis than virus-negative ones. This
means that early diagnosis of the presence of a viral myocardial
infection improves the patient's prognosis [64].
In this study, we did not have consistent data on the value of the
parameters of the cardiac biomarkers Troponin I and T as well as
NT-pro BNP, which is an objective shortcoming of this study. Also at
that time we did not routinely do a left atrial volume index (LAVI)
which is a better indicator of diastolic function than the left
atrial size. Echocardiography of myocardial deformation using
speckle tracking technology (myocardial strain) will provide a
stronger echo tool in the evaluation of clinically suspected
myocarditis.
CONCLUSION
Diagnosis of acute viral myocarditis is not easy to make and is
based on the criteria for clinically suspected myocarditis of the
European Society of Cardiology (ESC), which include clinical
presentations and 4 different diagnostic categories, with a dominant
role of ECG and echocardiography in everyday clinical practice with
necessary exclusion of other cardiovascular diseases. The whole
group of clinically suspected myocarditis A had highly statistically
significantly lower parameters of systolic and diastolic function
compared to control group B. Diastolic left ventricular dysfunction
dominated in 31% where 17 patients had severe diastolic dysfunction
grade III and clinically heart failure with preserved ejection
fraction. Regional systolic dysfunction was found in 17% and global
left ventricular systolic dysfunction (EF <50%) in 15% with left
ventricular dilatation and criteria for inflammatory cardiomyopathy.
Changes in myocardial texture - hyperechoic myocardial zone and
signs of fibrosis - cicatrix were present in 13% of subjects, and a
highly significant increase in left ventricular telediastolic
dimension, myocardial mass index and left atrial size. 24 (19%)
patients had a normal echocardiographic finding, but with clinical
and ECG criteria for myocarditis. However, 81% of patients had some
of the echocardiographic pathological changes, which are more
specific for diagnosis than ECG changes. A normal ECG and
echocardiographic findings cannot be used to rule out a diagnosis of
myocarditis. Comparison of subgroups with the presence of antiviral
IgM antibody titer dynamics (A1) and without it (A2) did not reveal
a statistically significant difference in echocardiographic
parameters. The sensitivity of IgM titer to cardiotropic viruses is
very low and should not be used in the routine diagnosis of
myocarditis.
REFERENCES:
- Ammirati E, Cipriani M, Moro C, Raineri C, Pini D, Sormani
P, et al. Registro Lombardo delle Miocarditi. Clinical
presentation and outcome in a contemporary cohort of pa-tients
with acute myocarditis: multicenter lombardy
regis-try.Circulation. 2018; 138(11):1088–1099.
doi:10.1161/CIRCULATIONAHA.118.035319
- Hosenpud JD, McAnulty JH, Niles NR. Unexpected myocar-dial
disease in patients with life threatening ar-rhythmias. Br Heat
J 1986;56(1):55-61. doi: 10.1136/hrt.56.1.55.
- Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE,
Howard DL, et al. Underlying causes and long-term survival in
patients with initially unexplained cardiomi-opathy. N Engl J
Med 2000;342(15):1077-84. doi: 10.1056/NEJM200004133421502.
- Maron BJ, Udelson JE, Bonow RO et al : Eligibility and
Disqualification Recommendations for Competitive Ath-letes With
Cardiovascular Abnormalities: Task Force 3: Hypertrophic
Cardiomyopathy, Arrhythmogenic Right Ventricular Cardiomyopathy
and Other Cardiomyopa-thies, and Myocarditis. Circulation
2015;132(22):e273-80. doi: 10.1161/CIR.0000000000000239.
- Harmon KG, Asif IM, Meleshewski JJ et al. Incidence and
etiology of sudden cardiac arrest and death in High school
Athletes in the United States. Mayo Clin Proc.
2016;91(11):1493-1502. doi: 10.1016/j.mayocp.2016.07.021. Epub
2016 Sep 28.
- Chandra N, Bastiaenen R, Papadakis M, Sharma S: Sudden
cardiac death in young athletes: Practical challenges and
diagnostic dilemmas. J Am Coll Cardiol. 2013;61(10):1027 2013.
doi: 10.1016/j.jacc.2012.08.1032.
- Dennert R, Crijns HJ, Heymans S. Acute viral myocarditis Eur
Heart J. 2008;29(17):2073-2082. doi: 10.1093/eurheartj/ehn296.
Epub 2008 Jul 9
- Global Burden of Disease Study C. Global, regional, and
national incidence, prevalence, and years lived with disa-bility
for 301 acute and chronic diseases and injuries in 188
countries, 1990-2013: a systematic analysis for the global
burden of disease study 2013.Lancet. 2015; 386(9995):743–800.
doi: 10.1016/S0140-6736(15)60692-4
- Leone O, Veinot JP, Angelini A, Baandrup UT, Basso C, Berry
G. at al. 2011 Consensus statement on endomyocar-dial biopsy
from the Association for European Cardiovas-cular Pathology and
the Society for Cardiovascular Pathol-ogy. Cardiovasc Pathol
2012;21(4):245–74. doi:10.1016/j.carpath.2011.10.001. Epub 2011
Dec 3.
- Kindermann I, Barth C, Mahfoud F, Ukena C, Lenski M, Yilmaz
A. at al. Update on myocarditis. J Am Coll Cardiol
2012;59(9):779–92. doi: 10.1016/j.jacc.2011.09.074.
- Caforio ALP, Pankuweit S, Arbustini E, Basso C,
Gimeno-Blanes J, et al. Current state of knowledge on aetiology,
di-agnosis, management, and therapy of myocarditis: a posi-tion
statement of the European Society of Cardiology Working Group on
Myocardial and Pericardial Diseases. Eur Heart J
2013;34(33):2636–48. 2648a-2648d. doi: 10.1093/eurheartj/eht210.
Epub 2013 Jul 3.
- Ammirati E, Frigerio M, Adler ED, Basso C, Birnie DH,
Brambatti M, at al. Management of Acute Myocarditis and Chronic
Inflammatory Cardiomyopathy: An Expert Con-sensus Document. Circ
Heart Fail. 2020;13(11):e007405.
doi:10.1161/CIRCHEARTFAILURE.120.007405. Epub 2020 Nov 12.
- Lakdawala NK, Stevenson LW and Loscalozo J. cardiomyo-pathy
and myocarditis. IN: Jameson JL, Kasper DL, Lomgo DL, Fauci AS,
Hauser SL, Loscalozo J, editors. Harrisson´s Principles of
Internal Medicine 20.th ed. New York: McGraw Hill; 2018.p.
1779-1797.
- Richardson P, Mc Kenna W, Bristow M, et al. Report of the
1995 World Health Ogranization/International Society and
Federation of Cardiology Task Force on the Definition and
Classification of cardiomyopathies. Circulation.
1996;93(5):841-2. doi: 10.1161/01.cir.93.5.841.
- Arbustini E, Agozzino M, Favalli V and Narula J.
Myocardi-tis. IN: Valentin Fuster, Robert A. Harrington, Jagat
narula, Zubin J. Eapen, editors. HURST´S The HEART14th ed. New
York: McGraw Hill; 2017.p. 1528-1560.
- Raukar NP,Cooper LT. Implications of SARS-CoV-2-Associated
Myocarditis in the Medical Evaluation of Ath-letes.Sports
Health. 2021;13(2):145-148. doi: 10.1177/1941738120974747. Epub
2020 Nov 17.
- Bhatia HS, Bui QM, King K, DeMaria A, Daniels LB.
Subclin-ical left ventricular dysfunction in COVID-19. Int J
Cardiol Heart Vasc, 2021;34:100770. doi:
10.1016/j.ijcha.2021.100770. Epub 2021 Mar 24.
- Rathore SS, Rojas GA, Sondhi M, Pothuru S, Pydi R,
Kanch-erla N. et al. Myocarditis associated with Covid-19
disease: A systematic review of published case reports and case
se-ries. Int J Clin Pract. 2021;e14470. doi: 10.1111/ijcp.14470.
- Ozieranski K, Tyminska A, Jonik S, Marcolongo R, Baritus-sio
A, Grabowski M.et al . Clinically Suspected Myocarditis in the
Course of Severe Acute Respiratory Syndrome Novel Coronavirus-2
Infection: Fact or Fiction? J Card Fail . 2021;27(1):92-96. doi:
10.1016/j.cardfail.2020.11.002. Epub 2020 Nov 6.
- Sala S, Peretto G, Gramegna M, Palmisano A, Villatore A,
Vignale D. ET AL. Acute myocarditis presenting as a re-verse
Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory
infection. Eur Heart J. 2020;41(19):1861-1862. doi: 10.1093/eurheartj/ehaa286.
- Leslie T Cooper and Kirk U. Knowlton, MYOCARDITIS IN:
IN:Zipes DP, Libby P, Bonow RO, Mann DL, Tomaselli GF, Braunwald
E. BRAUNWALD’S HEART DISEASE: A TEXT-BOOK OF CARDIOVASCULAR
MEDICINE 11th ed. Phila-delphia: Elsevier; 2019 p 1617-1630.
- Sanguineti F, Garot P, Mana M, et al. Cardiovascular
mag-netic resonance predictors of clinical outcome in patients
with suspected acute myocarditis. J Cardiovasc Magn Re-son.
2015;17(1):78. doi: 10.1186/s12968-015-0185-2.
- Teele SA, Allan CK, Laussen PC, et al.: Management and
outcomes in pediatric patients presenting with acute ful-minant
myocarditis. J Pediatr. 2011;158(4):638-643.e1. doi:
10.1016/j.jpeds.2010.10.015.
- Mlczoch E, Darbandi-Mesri F, Luckner D, Salzer-Muhar U:
NT-pro BNP in acute childhood myocarditis. J Pediatr. 2012;
160(1):178-9. doi: 10.1016/j.jpeds.2011.08.065.
- Caforio AL, Tona F, Bottaro S, et al.: Clinical implications
of anti-heart autoantibodies in myocarditis and dilated car-diomyopathy.
Autoimmunity. 2008;41(1):35-45. doi: 10.1080/08916930701619235.
- Mahfoud F, Gartner B, Kindermann M, et al.: Virus serology
in patients with suspected myocarditis: Utility or futility?.
Eur Heart J. 2011;32(7):897-903. doi: 10.1093/eurheartj/ehq493.
- Marwick TH, De Maria AN, Blanchard DG and Zoghbi WA.
Echocardiography, Dilated cardiomyopathy. IN: Fuster V,
Harrington RA, Narula J, Eapen ZJ, editors. HURST´S The
HEART14th ed. New York: McGraw Hill; 2017.p. 353-432.
- Vojkan Čvorović i Ivan Stanković. Transezofagijalna
eho-kardiografija IN: Ivan Stanković, Aleksandar N. Nešković,
Zorica Mladenović editors. Klinička ehokardiografija 1th ed.
Beograd: ECHOS; 2021. p.477-490.
- Escher F, Kasner M, Kühl U, Heymer J, Wilkenshoff U, Tschöpe
C, Schultheiss HP. New echocardiographic find-ings correlate
with intramyocardial inflammation in en-domyocardial biopsies of
patients with acute myocarditis and inflammatory cardiomyopathy.
Mediators Inflamm. 2013;2013:875420. doi: 10.1155/2013/875420.
Epub 2013 Mar 20.
- Kasner M, Aleksandrov A, Escher F, Al-Saadi N, Makowski M,
Spillmann F, at al. Multimodality imaging approach in the
diagnosis of chronic myocarditis with preserved left ventricular
ejection fraction (MCpEF): The role of 2D speckle-tracking
echocardiography. Int J Cardiol. 2017;243:374-378. doi:
10.1016/j.ijcard.2017.05.038.
- Caspar T, Fichot M, Ohana M, El Ghannudi S, Morel O, Ohlmann
P. Late Detection of Left Ventricular Dysfunction Using
Two-Dimensional and Three-Dimensional Speckle-Tracking
Echocardiography in Patients with History of Nonsevere Acute
Myocarditis. J Am Soc Echocardiogr. 2017;30(8):756-762. doi:
10.1016/j.echo.2017.04.002. Epub 2017 Jun 7.
- Uziębło-Życzkowska B, Mielniczuk M, Ryczek R, Krzesiński P.
Myocarditis successfully diagnosed and controlled with speckle
tracking echocardiography. Cardiovasc Ultra-sound.
2020;18(1):19. doi: 10.1186/s12947-020-00203-4.
- Trifunović-Zamaklar D, Gordana Krljanac. Analiza
defor-macije miokarda. IN: Ivan Stanković, Aleksandar N.
Nešković, Zorica Mladenović editors. Klinička ehokardio-grafija
1th ed. Beograd: ECHOS; 2021. p.421-436.
- Aquaro GD, Perfetti M, Camastra G, Monti L, Dellegrottaglie
S, Moro C, at al. Cardiac MR with late gadolinium en-hancement
in acute myocarditis with preserved systolic function: ITAMY
study. J Am Coll Cardiol. 2017; 70(16):1977–1987. doi:
10.1016/j.jacc.2017.08.
- Gräni C, Eichhorn C, Bière L, Murthy VL, Agarwal V, Kaneko
K, at al. Prognostic value of cardiac magnetic reso-nance tissue
characterization in risk stratifying patients with suspected
myocarditis. J Am Coll Cardiol. 2017;70(16):1964–1976. doi:
10.1016/j.jacc.2017.08.050.
- Frustaci A, Russo MA, chimenti C. Randomized study on the
efficacy of immunosupressive therapy in patients with
virus-negative inflammatory cardiomyopathy: the TIMIC study. Eur
Heart J. 2009;30(16):1995-2002. doi: 10.1093/eurheartj/ehp249.
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A,
Ernande L et al. Recommendations for cardiac chamber
quantification by echocardiography in adults: an update from the
American Society of Echocardiography and the European
Association of Cardiovascular Imaging. Eur Heart J Cardiovasc
Imaging 2015;16(3):233–70. doi: 10.1093/ehjci/jev014.
- Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish
H, Edvardsen T et al. Recommendations for the evaluation of left
ventricular diastolic function by echo-cardiography: an update
from the American Society of Echocardiography and the European
Association of Cardi-ovascular Imaging. Eur Heart J Cardiovasc
Imaging 2016;17(12):1321–60. oi: 10.1093/ehjci/jew082.
- Mitchell C, Rahko PS, Blauwet LA et al. Guidelines for
Performing a Comprehensive Transthoracic Echocardio-graphic
Examination in Adults: Recommendations from the American Society
of Echocardiography . 2019;32(1):1-64. doi:
10.1016/j.echo.2018.06.004. Epub 2018 Oct 1.
- Dušan Bastać, Radosava Cvjetan i Angelina Stevanović.
Izvođenje ehokardiografskog pregleda. IN: Ivan Stanković,
Aleksandar N. Nešković, Zorica Mladenović editors. Klin-ička
ehokardiografija 1th ed. Beograd: ECHOS; 2021. p.23-40.
- Tschöpe C, Cooper LT, Torre-Amione G, Linthout S. Man-agement
of Myocarditis-Related Cardiomyopathy in Adults. Circulation
Research. 2019;124(11):1568–1583. doi:
10.1161/CIRCRESAHA.118.313578.
- Kindermann I, Kindermann M, Kandolf R, et al.:Predictors of
outcome in patients with suspected myocarditis. Circula-tion
2008:118(6):639-48.. doi: 10.1161/CIRCULATIONAHA.108.769489.
- Towbin JLA, Colan S. et al. Incidence, causes and outcome of
dilated cardiomyopathy in children. JAMA.
2006;296(15):1867-1876. doi: 10.1001/jama.296.15.1867.
- Schultheiss HP, Piper C, Sowade O, et al. Betaferon in
chronic viral cardiomyopathy (BICC) trial: Effects of inter-feron-β
treatment in patients with chronic viral cardiomy-opthy. Clin
Res Cardiol. 2016;105(9):763-73. doi: 10.1007/s00392-016-0986-9.
- Kuhl U, Paushchinger M, Seeberg B, et al. Viral persistence
in the myocardium is associated with progressive cardiac
dysfunction. Circulation. 2005;112(13):1965-1970. doi:
10.1161/CIRCULATIONAHA.105.548156.
- Kuhl U, Lassner D, von Schlippenback J, et al.
Interferon-Beta improves survival in enterovirus-associated
cardio-myopathy. J Am Coll Cardiol. 2012;60(14):1295-1296. doi:
10.1016/j.jacc.2012.06.026.
- Cihakova D, Rose NR. Pathogenesis of myocarditis and dilated
cardiomyopathy. Adv Immunol. 2008;99:95-114. doi:
10.1016/S0065-2776(08)00604-4.
- Anzini M, Merlo M, Sabbadini G, et al. Long-term evolution
and prognostic stratification of biopsy-proven active
myo-carditis.Circulation. 2013;128(22):2384-94. doi:
10.1161/CIRCULATIONAHA.113.003092.
- Caforio A, Calabrese F, Angelini A, et al. A prospective
study of biopsy-proven myocarditis:prognostic relevance of
clinical and aetipathogenetic features at diagnosis. Eur Heart
J. 2007;28(11):1326-33. doi: 10.1093/eurheartj/ehm076.
- Thor Edvardsen : Cardiomyopathies, myocarditis and the
transplanted heart IN John Camm et al. editors. ESC Text-book of
Cardiovascular Medicine, 3rd ed. 2019. p.457-460.
- Lancellotti P, Price S, Edvardsen T, Cosyns B, Neskovic AN,
Dulgheru R, at al. The use of echocardiography in acute
cardiovascular care: recommendations of the European Association
of Cardiovascular Imaging and the Acute Car-diovascular Care
Association. Eur Heart J Cardiovasc Imag-ing 2015;16(2):119–46.
doi: 10.1093/ehjci/jeu210.
- Peter Liu and Kenneth L. Baughman. Myocarditis IN Robert O.
Bonow, Douglas L. Mann Douglas P. Zipes, Peter Libby editors.
BRAUNWALD’S HEART DISEASE: A TEXT-BOOK OF CARDIOVASCULAR
MEDICINE. Philadelphia 9.th ed. 2012 p.1595-1610.
- Kuhl U, Pauschinger M, Noutsias M, et al.: High prevalence
of viral genomes and multiple viral infections in the
myo-cardium of adults with “idiopathic” left ventricular dys-function.
Circulation. 2005;111(7):887-93. doi:
10.1161/01.CIR.0000155616.07901.35.
- Cavalcante JL, Marek J, Sheppard R, Starling RC, Mather PJ,.
Alexis JD at al. Diastolic function improvement is associat-ed
with favourable outcomes in patients with acute non-ischaemic
cardiomyopathy: insights from the multicentre IMAC-2 trial Eur
Heart J Cardiovasc Imaging. 2016;17(9):1027–35. doi: 10.1093/ehjci/jev311.
- Pinamonti B, Alberti E, Cigalotto A, Dreas L, Salvi A,
Silves-tri F, at al. Echocardiographic findings in myocarditis.
Am J Cardiol. 1988;62(4):285-91. doi:
10.1016/0002-9149(88)90226-3.
- Grun S, Schumm J, Greulich s, et al. Long-term follow-up of
biopsy-proven viral myocarditis: predictors of mortality and
incomplete recovery. J am Coll cardiol. 2012;59(18):1604-15. doi:
10.1016/j.jacc.2012.01.007.
- Abbate A, Sinagra G, Bussani R, et al. Apoptosis in patients
with acute myocarditis. Am J Cardiol. 2009;104(7):995-1000. doi:
10.1016/j.amjcard.2009.05.041.
- Kim G, Ban GH, Lee HD, Sung SC, Kim H, Choi KH. Left
ventricular end-diastolic dimension as a predictive factor of
outcomes in children with acute myocarditis. Cardiol Young
2017;27(3):443-451. doi: 10.1017/S1047951116000706. Epub 2016
May 26.
- McCarthy 3rd RE, Boehmer JP, Hruban RH, Hutchins GM, Kasper
EK at al. Long-term outcome of fulminant myocar-ditis as
compared with acute (nonfulminant) myocarditis. N Engl J Med
2000;342(10):690-5. DOI: 10.1056/NEJM200003093421003.
- Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF.
Systematic review of patients presenting with suspected
myocardial infarction and nonobstructive coronary arter-ies.
Circulation. 2015; 131(10):861–70. doi:
10.1161/CIRCULATIONAHA.114.011201.
- Ivanova SK, Angelova SG, Stoyanova AS, Georgieva IL,
Nikolaeva-Glomb LK et al. Serological and Molecular Bio-logical
Studies of Parvovirus B19, Coxsackie B Viruses, and Adenoviruses
as Potential Cardiotropic Viruses in Bulgar-ia. Folia Med
(Plovdiv) 20161;58(4):250-256. doi: 10.1515/folmed-2016-0036
- Younis A, Matetzky S, Mulla W, Masalha E, Afel Y,
Cherno-mordik F, Fardman A, Goitein O, Ben-Zekry S, Peled Y, et
al.. Epidemiology characteristics and outcome of patients with
clinically diagnosed acute myocarditis.Am J Med.
2020;133(4):492–499. doi: 10.1016/j.amjmed.2019.10.015
- White JA, Hansen R, Abdelhaleem A, Mikami Y, Peng M, Rivest
S, Satriano A, et al.. Natural history of myocardial in-jury and
chamber remodeling in acute myocarditis.Circ Cardiovasc Imaging.
2019;12(7):e008614. doi: 10.1161/CIRCIMAGING.118.008614.
- Chen WH, Guo YS, Zhang DH and Zhang HJ. Long-Term Prognosis
of Suspected Myocarditis and Cardiomyopathy Associated with
Viral Infection of the Myocardial Tissue: A Meta-Analysis of
Cohort Studies. Cardiovasc Ther. 2019;2019:9342792. doi:
10.1155/2019/9342792.
|
|
|
|









