| |
|
|
INTRODUCTION AND
SIGNIFICANCE OF THE PROBLEM
The new 2019 European Society of Cardiology (ESC) guide for the
diagnosis and management of chronic coronary syndromes [1] focuses
on the new comprehensive term Chronic Coronary Syndromes (CCS) for
all forms of chronic coronary artery disease (CCAD) except acute
coronary syndromes [2,3], rather than only stable coronary artery
disease (SCAD), as a previous ESC guide from 2013 [4]. The new guide
of the European Association of Cardiologists from 2019 primarily
brings a paradigm shift for stable coronary heart disease to the
comprehensive term chronic coronary syndromes (CCS), which
essentially means that CAD has complex clinical scenarios and can
have periods of instability at any evolutionary stage. Essentially,
the clinical presentation of coronary heart disease is categorized
into either acute coronary syndromes (ACS) [2,3,5] or chronic
coronary syndromes (CCS) [1]. Coronary heart disease (CAD) is a
dynamic pathological process of appearance and growth of
atherosclerotic plaques in epicardial coronary arteries, but also in
their smaller intramyocardial branches [6,7, 8,9] (microvascular
disease) with or without coronary vasospasm [10-13], without whether
they are functionally fixed obstructive (stenotic) or
non-obstructive [14,15]. This dynamic process leads to a functional
alteration of the coronary blood flow or myocardial ischemia.
Myocardial ischemia can be reduced, stabilized or stagnation or
regression of atherosclerotic plaques can be achieved through
therapeutic interventions: optimal non-invasive medical ie. (medical
therapy-OMT) which consists of lifestyle changes, reduction of risk
factors and optimal pharmacotherapy (OPhT) and optimal invasive
interventions - percutaneous or surgical myocardial
revascularization (RM). [16-21]. CAD has long stable periods, but
may also become unstable at some period due to acute
atherothrombotic events — breakup or erosion of atherosclerotic
plaque. However, the disease is chronic, most often progressive and
therefore serious, even in clinically asymptomatic periods [1]
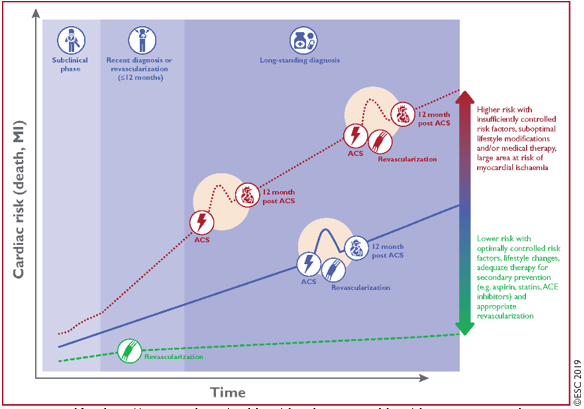
(FIGURE 1).FIGURE 1: CLINICAL PRESENTATION -
CLINICAL SCENARIOS OF CHRONIC CORONARY SYNDROMES
Retrieved from
https://www.escardio.org/Guidelines/Clinical-Practice-Guidelines/Chronic-Coronary-Syndromes
The dynamic nature of the CAD process results in different
clinical presentations or clinical scenarios, acute coronary
syndromes (ACS) [2,3,5] or chronic coronary syndromes (CCS) [1].
CLINICAL PRESENTATION - CLINICAL SCENARIOS OF CHRONIC CORONARY
SYNDROMES (CCS).
The clinical presentation of CCS consists of 6 leading and most
common clinical scenarios [1]:
1.Patients with suspected CAD and stable angina pectoris (AP) and /
or dyspnea on exertion.
2. Patients with newly developed heart failure (HF) or left
ventricular dysfunction (LVD) and suspected CAD.
3. Asymptomatic and symptomatic patients with stabilized symptoms up
to 1 year after ACS or myocardial revascularization (RM).
4. Asymptomatic and symptomatic patients more than 1 year after ACS
orRM.
5. Patients with AP and suspected vasospastic or microvascular
disease.
6. Asymptomatic individuals in whom CAD was detected at screening.
Each of these scenarios is classified as CCS, is a consequence of
different evolutionary phases of CAD and has a different risk for
future adverse CV events (death or myocardial infarction) and this
risk may change over time [1].
NEW CONCEPTS AND RECOMMENDATIONS FOR CCS
New concepts and recommendations for CCS and revised concepts and
recommendations from the previous ESC guide in 2013 [4], in this
2019 ESC guide [1], based on current available evidence from a large
number of randomized studies, registers and expert consensus (cited
a huge number of scientific papers: 529 references) have a holistic
approach, give quite clear guidelines for the diagnosis and therapy
of CCAD and systematically process all clinical presentations of
CCAD in a clear and clinically applicable way. This ESC Guide and
its recommendations should facilitate clinical decision-making by
physicians in their day-to-day practice [1].
NEW MAIN RECOMMENDATIONS OF CLASS I (There is evidence and / or
general agreement that a given treatment or procedure is benefitial,
useful and effective: Wording to use: Is recommended or is
indicated)
1. Non-invasive functional imaging diagnostic test for the detection
of myocardial ischemia or coronary MSCT angiography should be the
initial test for the diagnosis of CAD in symptomatic patients in
whom obstructive coronary heart disease cannot be ruled out by
clinical judgment alone.
2. It is recommended that the selection of the optimal test for the
diagnosis of CAD be based on the Clinical Probability of CAD and
other patient characteristics that affect test performance, local
availability, and expertise.
3. Non-invasive functional imaging test for myocardial ischemia is
recommended if coronary MSCT angiography shows CAD of uncertain
functional significance or is non-diagnostic, inclusive.
4. Invasive coronary angiography (ICA) is recommended as an
alternative test for the diagnosis of coronary artery disease (CHD)
in patients with high clinical probability and severe symptoms
refractory to medical therapy (nonpharmacological and
pharmacological) or typical angina at low exercise and when clinical
evaluation indicates at high risk of adverse CV events. Invasive
functional assessment (FFR, iwFR) must be available and used to
evaluate stenosis prior to coronary revascularization, except in the
case of a very high degree of coronary stenosis ≥90% of the stenosis
diameter.
5. In patients with atrial fibrillation (AF) with a CHA2DS2-VASc
score ≥ 2 for males and ≥ 3 for females, non-vitamin K antagonists (NOAC,
DOAC) are preferred if there are no contraindications.
6. After percutaneous coronary revascularization (postPCI) in
patients with AF, NOACs: Apixaban 2 x 5 mg, Dabigatran 2 x150 mg,
Edoxaban 60 mg and Rivaroxaban 20 mg once daily have an advantage
over vitamin K antagonists (VKA) in combination with in combination
with antiplatelet therapy (mono- or dual-DAPT at high hemorrhagic
risk).
7. Proton pump inhibitors are recommended in patients at high risk
of gastrointestinal bleeding, according to the HAS-BLED score, in
the following subgroups: patients with aspirin monotherapy, dual
antiplatelet therapy (DAPT) or oral anticoagulant monotherapy.
8. If the target value of serum LDL cholesterol is not reached with
the maximum dose of statins, combination with ezetimibe is
recommended, and in VERY HIGH RISK, a third drug PCSK9-inhibitor (Proprotein
convertase subtilisin / kexin type 9) is added parenterally.
9. Sodium glucose-2-cotransporter inhibitors (SGLT2-I):
empagliflozin, canagliflozin or dapagliflozin are recommended in
patients with diabetes mellitus (DM) and CCS and cardiovascular
disease.
10. Glucagon-like peptide-1 (GLP-1) receptor agonists liraglutide or
semaglutide are recommended in patients with DM and CCS and
cardiovascular disease.
NEW AND / OR REVISED CLASS IIa MAIN RECOMMENDATIONS (There is
conflicting evidence and / or divergence of opinion about the
usefulness / efficacy of a given treatment or procedure, but weight
of evidence/opinion favor of usefulness / efficacy. Wording to use:
Should be considered)
1. Invasive coronary angiography with the availability of invasive
functional evaluation should be considered to confirm the diagnosis
of CAD in patients with an uncertain diagnosis on noninvasive tests.
2. Coronary MSCT angiography should be considered as an alternative
to invasive coronary angiography if other non-invasive tests are
ambiguous or non-diagnostic.
3. The addition of another antiplatelet drug to aspirin for
long-term secondary prevention should be considered in patients with
a high ischemic risk and without a high risk of bleeding.
4. Long-term oral anticoagulant therapy (OAC) should be considered
with AF and CHA2DS2-VASc = 1 for males and 2 for females,
non-vitamin K antagonists (NOAC) are preferred, if there are no
contraindications
5. In patients with AF and NOAC, where the risk of haemorrhagic risk
outweighs the risk of stent thrombosis or ischemic stroke, a lower
dose of NOAC should be given (Rivaroxaban 15 mg once daily or
Dabigatran 2 x 110 mg in combination with mono or double
antiplatelet therapy).
6. In post-PCI patients with AF or other indications for OAC, triple
therapy with aspirin, clopidogrel, and OAC should be considered for
at least one month or longer when the risk of stent thrombosis
outweighs the haemorrhagic risk, with a total duration of up to 6
months. both risks and is clearly stated on discharge from the
hospital!
7. Angiotensin converting enzyme (ACEI) inhibitors should be
considered in CCS patients at very high risk of adverse
cardiovascular events.
8. Ranolazine, nicorandil, ivabradine and trimetazine are converted
to IIa (from class IIb-utility / efficacy is much less based on
evidence / views).
CLASS III MAIN RECOMMENDATIONS. There is evidence and / or general
agreement that a given treatment or procedure is not useful/
effective and in some cases may be harmful: Wording to use: Is not
recommended
1. Coronary MSCT / MDCT angiography is not recommended when there
are extensive coronary calcifications, irregular heart rate,
significant obesity, inability of the patient to hold his breath
long enough and any other factors that would affect the failure to
obtain a quality image.
2. Changes in the ST segment of the ECG during PSVT should not be
used as evidence of CAD.
3. Outpatient ECG monitoring (Holter ECG) should not be routinely
used in the examination of patients with suspected CCS.
4. Coronary calcium score via MSCT is not recommended for
identification of persons with obstructive CAD.
5. Exercise ECG test (ergometric ECG stress test on a treadmill or
bicycle) in patients with ≥0.1Mv (1mm) ST segment depression on an
ECG at rest, or digitalis treatment is not recommended for
diagnostic purposes of CCS.
6. Invasive coronary angiography (ICA) is not recommended as the
only method for risk stratification in CCS.
7. Nitrates are not recommended for the treatment of CCS in patients
with hypertrophic obstructive cardiomyopathy or in concomitant
therapy with phosphodiesterase inhibitors (Sildenafil et al.).
8. The use of ticagrelor or prasugrel is not recommended as part of
triple antithrombotic therapy with acetyl-salicylic acid (ASA) and
oral anticoagulant therapy (OAC).
9. Coronary MSCT angiography is not recommended as a routine test to
monitor patients diagnosed with chronic coronary syndrome (CCS).
10. Carotid echosonography with determination of intimomedial layer
thickness is not recommended for CCS risk stratification.
11. In low-risk asymptomatic adult non-diabetics, coronary MSCT
angiography or functional imaging tests for ischemia are not
indicated for further diagnostic evaluation.
12. Routine determination of circulating cardiac biomarkers is not
recommended for stratification of cardiovascular risk in patients
with CCS.
13. The combination of drugs from the ACEI and ARB groups is not
recommended for CCS.
14. In severe heart valve disease, stress stress tests should not be
used routinely to detect CAD, due to low diagnostic benefit and
potential risk of complications.
15. Sex hormone replacement therapy is not recommended for risk
reduction in postmenopausal women.
16. Transmyocardial revascularization is not recommended for
patients with severe AP refractory to optimal medical treatment (OMT)
and myocardial revascularization (RM) strategies.
SCENARIO 1: PATIENTS WITH SUSPECT CAD-CCS AND STABLE AP and / or
EFFORT DYSPNEA.
The procedure (algorithm) in 6 steps in the approach to initial care
of patients with suspected CCS and stable AP or dyspnea on exertion
is given in Figure 2.
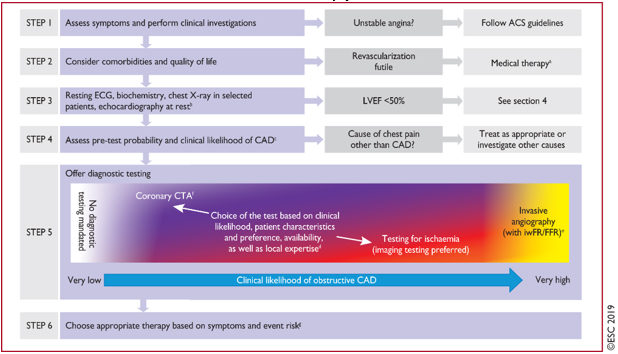
FIGURE 2. 6-step procedure (algorithm) in the
approach to initial care of patients with suspected CCS and stable
AP or dyspnea
Retrieved from
https://www.escardio.org/Guidelines/Clinical-Practice-Guidelines/Chronic-Coronary-Syndromes
Instead of the previous 3 steps according to the ESC guide from
2013 [4,22], a procedure or algorithm has now been introduced in 6
STEPS [1] in the approach to initial care of patients with suspected
CCS:
STEP 1: Assessment of symptoms (TABLE 1) uses the traditional
clinical classification of suspected anginal symptoms: chest
discomfort - discomfort (pain) on exertion usually shorter than 10
minutes (pain lasting seconds is usually not anginal) and conducting
clinical trials, identifying patients with unstable angina and other
forms of ACS. AP can paradoxically decrease with further effort
(walk-through angina) or with the next effort (warm-up angina) [23].
TABLE 1. The traditional clinical classification
of suspected anginal symptoms: chest discomfort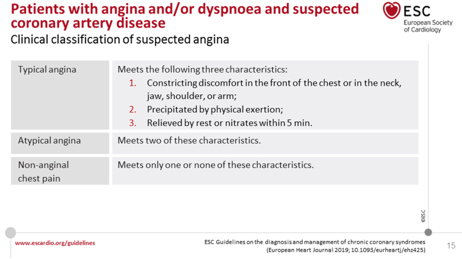
It should not be emphasized how important it is to quickly rule
out other acute acute cardiac conditions: acute coronary syndrome
(ACS) -unstable angina pectoris- identical pain as in AP but
lasting> 20 minutes. One should always think of a dissecting aortic
aneurysm, ie Acute aortic syndrome (AAS), pulmonary embolism,
pericarditis and myocarditis. In the differential diagnosis,
consider non-cardiac diseases that may resemble anginal pain. The
most common diseases that can mimic angina pectoris:
gastroesophageal diseases (40%), thorax wall syndromes (Costochondritis
and Titze syndrome), some lung diseases, pneumothorax, pleuritis and
herpes zoster intercostalis
STEP 2- Consider the general condition and condition of the patient,
assess the quality of life and the presence of comorbidities that
potentially affect the therapeutic decision. If performance of load
tests and coronary revascularization are unlikely due to the general
condition, immediately introduce OMT, especially antianginal
pharmacological therapy.
STEP 3. Basic clinical supplementary trial.
Includes basic examination: electrocardiogram (ECG), in selected
patient ambulatory ECG Holter monitoring, biochemical analysis,
radiography of the thorax in selected patients. An ECG is crucial
for the diagnosis of myocardial ischemia, typically a reversible
horizontal depression of the ST segment in two or more adjacent ECG
leads during or immediately after an anginal attack. The descending
ST-segment depression is less specific and the slow-ascending
depression is the least specific for the diagnosis of ischemia,
while the fast-ascending ST-segment depression is a normal variant
in tachycardia [24]; Holter ECG often reveals asymptomatic
myocardial ischemia in the form of horizontal depression of the ST
segment on exertion [24,25,26]; The ECG may also indicate indirect
signs of CAD: pathological Q tooth [27]; left bundle branch block (LBBB)
or atrioventricular (AV) blocks, extrasystoles [27]; In an episode
of atrial fibrillation (AF) with asymptomatic myocardial ischemia -
ST depression [28,29]. In contrast to AF, ST depression during
paroxysmal supraventricular tachycardia (PSVT) is not predictive of
ischemia. Echocardiographic assessment of left ventricular function
(LV), primarily left ventricular ejection fraction (EF), is
mandatory. When the EF is <50% the patient is referred directly for
invasive coronary angiography (ICA). Transthoracic echocardiography
(TTE) as the single most informative diagnostic method in cardiology
has a crucial role in excluding alternative causes of chest
discomfort [30] and for risk stratification. In the case of
suboptimal echo imaging (<10% of cases), transesophageal
echocardiography (TOE) and cardiomagnetic resonance imaging (CMR)
are used [31].
STEP 4 Assessment of pre-test probability and clinical probability
of CAD-CHD
The European Guide 2019 gives increased and renewed importance to
the determination of pre-test probability (PTP) of obstructive
coronary heart disease, but the classic PTP, Diamond and Forrester
based on age, sex and nature of symptoms [31] have undergone major
changes based on new evidence [32]. The mean PTP is 15% to 85%.
Using the new table (TABLE 2) reduces the overestimation of the
incidence of coronary heart disease. [1, 31, 32].
TABLE 2. NEW REVISED PRESTEST PROBABILITY
Foldyna B, Udelson JE, Karady J, et al. insights from the PROMISE
trial. Eur Heart J Cardiovasc Imaging 2018; 20:574 581.
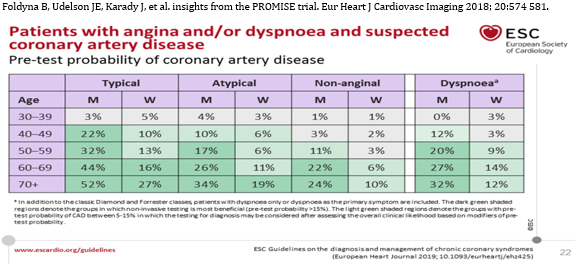
Retrieved from
https://www.escardio.org/Guidelines/Clinical-Practice-Guidelines/Chronic-Coronary-Syndromes
A new term, Clinical Probability of Obstructive Coronary Disease
(CPCAD), is introduced, which uses different risk factors for CAD as
modifiers of PTP probability. Reduction of probability for
obstructive CAD: normal ECG test with physical load and normal
calcium score of coronary arteries (Agatston = 0) [1, 33]. Factors
that increase CPCAD:
A) dyslipidemia, diabetes, hypertension, smoking, family history of
CAD and sudden death.
B) Changes in the ECG at rest: Q wave and changes in the ST segment
and T wave.
C) Left ventricular dysfunction referring to CAD
D) Abnormal exercise ECG test
E) increased calcium score by CT
The selection of the initial non-invasive diagnostic test
(functional or anatomical image) is based on PTP or CPCAD.
STEP 5. Selection of the optimal diagnostic test for diagnosing CAD
Selection of the optimal diagnostic test for diagnosing CAD, based
on patient profile, local availability and expertise. [1, 36-42] is
shown in Figure 3.
Figure 3. MAIN DIAGNOSTIC PATHWAYS IN SYMPTOMATIC
PATIENTS WITH SUSPECT OBSTRUCTIVE CAD CCS
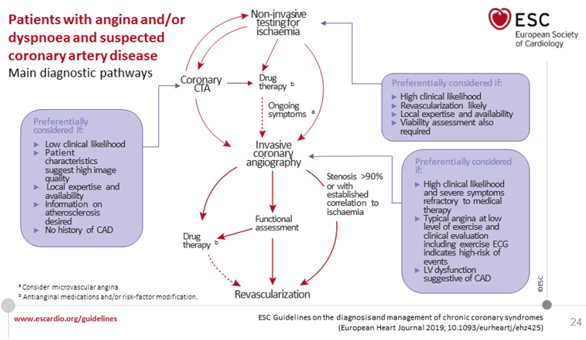
Retrieved from
https://www.escardio.org/Guidelines/Clinical-Practice-Guidelines/Chronic-Coronary-Syndromes
In patients in whom revascularization is “futile” due to
comorbidity and overall quality of life (STEP 2), the diagnosis of
CAD can be made clinically and only OMT- optimal medical therapy is
required. If the diagnosis of CAD is uncertain, making a diagnosis
using non-invasive functional tests to record myocardial ischemia
before treatment is a reasonable option; on the other hand in a
patient with a high clinical probability of CAD, when symptoms have
not responded to medical therapy or severe typical low-grade angina
is present and / or initial clinical assessment (including
echocardiogram and in selected patients ECG exercise test or
Ergometric ECG test) indicates a high risk of adverse events, switch
directly to invasive coronary angiography (ICA) without further
diagnostic testing. Under such circumstances, the indication for MR
should be based on appropriate invasive confirmation of the
hemodynamic significance of the stenosis: FFR, CFR [43, 44].
Existing guidelines recommend the use of either noninvasive
functional imaging imaging of ischemia or anatomical imaging using
coronary CT angiography (CTA) as an initial test for the diagnosis
of CAD. Functional non-invasive ischemia tests for the diagnosis of
obstructive CAD are designed to detect myocardial ischemia by ECG
changes, irregularities of wall movement using CMR stress or stress
echocardiography, or perfusion changes by myocardial scintigraphy (SPECT),
positron emission cardiography or contrast CMR. Ischemia can be
caused by physical exertion (erggometrically) or pharmacological
stressors, either through increased myocardial function and oxygen
demand, or by heterogeneity in myocardial perfusion by vasodilation.
Non-invasive functional tests have high accuracy for detecting
coronary stenosis that restricts flow compared to invasive
functional examination by fractional flow reserve (FFR) [45].
However, insignificant coronary stenoses and atherosclerotic plaques
not associated with ischemia remain undetected by functional testing
and in the presence of a negative functional test, patients should
receive risk factor modification based on ESC recommendations for CV
prevention [6].
Anatomical non-invasive assessment
Anatomical noninvasive assessment by visualization of coronary
arteries, imaging and lumen of the coronary artery wall can be
reported using intravenous contrast agent by coronary CT angiography
(CTA), which provides high accuracy for detecting obstructive
coronary stenoses, as well as invasive coronary angiography [45A]
the recordings are based on anatomy. However, stenoses that amount
to 50 to 90% by visual examination are not necessarily functionally
significant, ie. they do not always cause myocardial ischemia.
[45.46]. Therefore, non-invasive or invasive functional testing is
recommended for further assessment of angiographic stenosis detected
by coronary CTA or ICA, unless high-grade stenosis (> 90% of
diameter) is detected by invasive angiography. The presence or
absence of non-obstructive coronary atherosclerosis on coronary CTA
provides prognostic information and can be used for preventive
therapy. [47].
The role of Exercise (ergo) ECG test
The ECG ergometric stress test has poorer diagnostic performance
compared to diagnostic visualization tests and has limited power to
rule out obstructive CAD. [45]. Therefore, these guidelines
recommend the use of diagnostic imaging tests instead of exercise
ECG as an initial test for the diagnosis of constructive CAD.
Exercise ECG test can be considered as an alternative for the
diagnosis of obstructive CAD, if visualization (imaging tests) are
not available, bearing in mind the risk of false negative and false
positive test results. [45]. Exercise ECG has no diagnostic value in
patients with ECG abnormalities that prevent the interpretation of
ST-segment changes.
Influence of clinical probability on the choice of diagnostic
test
Each non-invasive diagnostic test has a certain range of clinical
probability for obstructive CAD where the usefulness of its
application is maximum. Test probability coefficients are a useful
parameter of their ability to properly classify patients and can be
used to facilitate the selection of the most useful test for any
patient. [45]. Given the clinical probability of obstructive CAD and
the probability coefficient of a particular test, a post-test
probability for obstructive CAD after performing such a test can be
assessed.
Using this approach, the optimal range of clinical probability for
each test can be estimated, where patients can be reclassified from
medium (15–85%) to any: low (<15%) or high probability of CAD> 85%
after the test [45]. Coronary CTA is preferred in patients with a
lower range of clinical probability of CAD (previously numerically
PTP 15-65%), without prior diagnosis of CAD and important conditions
associated with a high probability of good image quality. Coronary
CTA detects subclinical coronary atherosclerosis, but can also rule
out anatomically and functionally significant CAD.
Non-invasive functional tests for ischemia have the advantage
because they directly show the area of myocardial ischemia by
provoking ischemia. Before revascularization, functional assessment
and [45]. schemes (non-invasive or invasive method) is required in
most patients.
In addition to diagnostic accuracy and clinical probability, the
choice of a non-invasive test depends on other patient
characteristics, local expertise, and test availability. Some
diagnostic tests may be better in some patients than others. For
example, tachyarrhythmia and the presence of extensive coronary
calcification are associated with an increased likelihood of
non-diagnostic quality of the coronary CTA image and are not
recommended in such patients. [51]. Stress echocardiography or SPECT
myocardial perfusion imaging may be combined with dynamic TFO and
may be desirable if additional information is available during the
TFO ECG test. TFP cannot be used for diagnostic purposes in the
presence of ECG abnormalities that prevent the assessment of
ischemia. The risks associated with different diagnostic tests must
be weighed for and against the benefit to the particular patient
[52]. Similarly, contraindications for pharmacological stressors and
contrast agents (iodine- and gadolinium-based contrast agents)
chelates) should be considered. When testing is used appropriately,
the clinical benefit of accurate diagnosis and therapy outweighs the
projected risks of testing itself [52].
Invasive examination
For strictly diagnostic purposes, ICA is required only in patients
suspected of having obstructive CB in the case of unconvincing,
ambiguous or inclusive non-invasive testing or, exceptionally, in
patients of certain public occupations of special importance
(drivers, pilots, machine workers, police officers). and the like),
due to security and regulatory issues. [53]. However, ICA may also
be necessary when a noninvasive assessment suggests a very high risk
of adverse events to determine revascularization options. [53]. In a
patient with a high clinical likelihood of CAD and symptoms not
responding to medical therapy or with typical low-effort angina, and
an initial clinical assessment indicating a high risk of events,
early ICA without prior noninvasive risk stratification may be a
reasonable solution to identify lesions that may be suitable for
myocardial revascularization (FIGURE 3). Invasive functional
assessment should complement the ICA, especially in patients with 50
to 90% coronary stenosis or multivessel disease, given the frequent
discrepancies in the angiographic and hemodynamic severity of
coronary stenosis. [53-58]. ICA should not be performed in patients
with angina who refuse invasive procedures and avoid
revascularization, who are not candidates for percutaneous coronary
intervention (PCI) or coronary artery bypass grafting (CABG), or in
whom myocardial revascularization is not expected to improve
functional status or quality of life
STEP 6 After the diagnosis of CCS is made, the risk of adverse
events is stratified by functional stress tests: isotopic
stress-rest SPECT, the most available pharmacological stress echo
dobutamine or dipyridamole and the least available cardiomagnetic
resonance (CMR) stress with dobutamine and contrast perfusion. The
decision on further treatment is based on determining the level of
risk [1]. Definition of risk levels according to annual mortality:
no ischemia, mortality from adverse events less than 1%; medium risk
- annual mortality between 1% and 3%; high annual mortality is over
3% (Table 3).
TABLE 3. DEFINITION OF HIGH RISK LEVEL (HIGH EVENT
RISK) FOR FUNCTIONAL IMAGE TESTS AND NON-INVASIVE ANATOMICAL CT
CORONARY ANGIOGRAPHY
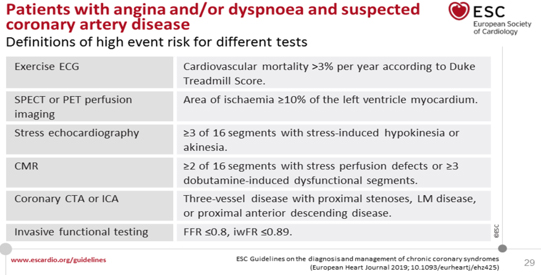
Figure 4. COMPARISON OF CV RISK LEVELS OF ASYMPTOMATIC PERSONS IN
PRIMARY PREVENTION OF ADVERSE CV EVENTS AND IN ESTABLISHED CCS IN
SECONDARY PREVENTION
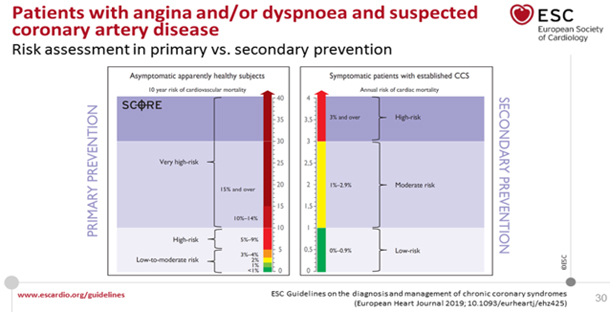
Retrieved from
https://www.escardio.org/Guidelines/Clinical-Practice-Guidelines/Chronic-Coronary-Syndromes
If the angina is very severe but not unstable, according to the
well-known Canadian Classification of the Canadian Class IV
Association (TABLE 4), with a pretest probability greater than 85%
according to Bayes' theorem, ICA is performed immediately without
previous noninvasive tests, but with coronary fractional flow
reserve assessment. (FFR) [45, 56, 59].
TABLE 4. Classification of severity of angina
pectoris and / or dyspnea on exertion according to the Canadian
Cardiovascular Association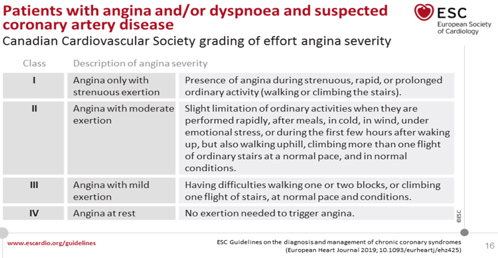
The role of coronary MSCT angiography is to rule out significant
disease in patients with a lower intermediate probability of 15 to
50%. Finally, the choice of adequate therapy is made: lifestyle
change, pharmacological therapy of CAD and myocardial
revascularization (MR), based on symptoms and risk of adverse CV
events.
NON-PHARMACOLOGICAL MEASURES IN THE TREATMENT OF CHRONIC CORONARY
SYNDROMES (for all ccs, especially for scenario 1)
The guide underlines the crucial role of a healthy lifestyle or
other preventive measures to reduce the risk of consequent
cardiovascular events and mortality [1], as shown in the essential
studies COURAGE [60] and FAME [44]. Regular taking of medications
for the treatment of hypertension, hyperlipidemia, diabetes, etc.
should ensure the achievement of target values of blood pressure,
LDL cholesterol, HDL, triglycerides and glycemia (HbA1c), which
leads to stagnation and regression of atherosclerosis, which is
discussed in detail in the ESC Guide for CV prevention 2016 [6].
The involvement of a multidisciplinary team in preventive work is
recommended: cardiologist, general practitioner (GP), nurses,
nutritionist, psychologist, psychotherapist and pharmacist (class I
evidence B) [1]. The use of a healthy lifestyle, as a preventive
intervention, reduces the risk of subsequent development of adverse
CV events and mortality. The application of healthy behavior is
important: smoking cessation, recommended physical activity, healthy
diet, maintaining a healthy weight, which significantly reduces the
risk of future cardiovascular events and death, and which is based
on evidence. [1, 61, 62]. The benefits are obvious as early as 6
months after the index event [1, 61, 62]. Primary health care plays
an important role in prevention, the EUROACTION study showed that a
program coordinated by a primary care nurse improves the reduction
of risk factors. [63].
Smoking cessation improves the prognosis in patients with HCV,
including a 36% reduction in the risk of death for those who
successfully quit. Measures to promote smoking cessation include
brief tips and advice on behavioral intervention and pharmacological
therapy including nicotine replacement. Patients should also avoid
passive smoking. Short doctor's advice doubles the likelihood of
smoking cessation in the short term, but more intensive advice and
support (behavioral interventions, telephone support or self-help
measures) is more effective than short advice, especially if
continued for one month [62,63]. All forms of nicotine replacement
therapy, bupropion, and varenicline are more effective in smoking
cessation than self-control; combining a behavioral and
pharmacological approach to smoking cessation is effective and
recommended. [64].
Healthy eating [65]: Diet rich in vegetables, fruits and whole
grains. Limit saturated fat intake to <10% of total intake. Limit
alcohol to <100 g / week or 15 g / day.
Healthy body mass gain and maintain a healthy mass (BMI <25 kg / m2)
or lose weight through recommended energy intake and increased
physical activity.
Obesity is associated with shorter overall life expectancy and
overweight is associated with the development of cardiovascular
disease (CVD) [66]. Waist circumference is a sign of central obesity
and metabolic syndrome [30] and is strongly associated with the
development of CVD and diabetes. The recommended waist circumference
is ≤94 cm for men and ≤80 cm for women. In people with CVD,
intentional weight loss is associated with a significantly lower
risk of adverse events [67].
Moderate alcohol intake (1-2 drinks per day) does not increase the
risk of AMI.
Physical activity. The exercise is called "pollypill" because of its
many beneficial effects on CV risk factors and the CV system [21,
68, 69, 70]. Physical activity reduces AP severity, improves oxygen
transport in the myocardium and increases exercise capacity, and is
an independent predictor of increased survival in men and women with
CCS [21, 68, 69, 70]. Every 1 mL / kg / min increase in peak oxygen
consumption was associated with a 14% reduction in CVD risk and an
all-cause cause of death in women and men. [21]. Recommendations for
physical activity for patients with CCS are 30 to 60 min of
moderate-intensity aerobic activity ≥5 days per week. [6, 69]. Even
irregular physical activity in leisure time reduces the risk of
mortality in previously seated patients [72] and increasing activity
is associated with lower CV mortality [73]. Strength exercises
maintain muscle mass and function, and in addition to aerobic
activity (fast walking, swimming, etc.), they give beneficial
effects in terms of lowering insulin resistance, lipid levels and
blood pressure.
CV rehabilitation based on physical exercise has constantly shown
efficacy in reducing CV mortality and hospitalizations compared to
the control group in patients with CAD and this benefit remains at
the present time [74,75,76, 77].
Psychosocial factors. Patients with CAD have a twice-increased risk
of depression and anxiety disorders compared to people without heart
disease [78]. Psychosocial stress, depression and anxiety are
associated with poorer CCS outcomes. Clinical trials have shown that
psychological (eg, counseling and / or cognitive-behavioral therapy)
and pharmacological interventions with psychopharmaceuticals have
had beneficial effects on depression, anxiety, and stress, with some
evidence of reduced cardiac mortality and adverse events compared
with placebo. [79,80,81].
Environmental factors. Air pollutants are estimated as one of the 10
leading risk factors for global mortality. Exposure to air pollution
also increases the risk of AMI as well as hospitalization and death
from HF, stroke and arrhythmias. [82]. Patients with CCS should
avoid areas with heavy traffic due to pollution and noise [82,83]
Sexual activity. Patients with CCS are often concerned about the CV
risk of sexual activity and / or sexual dysfunction. [84,85]. The
risk of causing sudden death or AMI is very small, especially when
sexual activity with a stable partner in a known environment is
stress-free or without excessive food or alcohol intake [86].
Although sexual activity transiently increases the risk of MI, it
causes only <1% of acute MI and <1.7% for sudden death during sexual
activity. [86]. Energy expenditure during sexual activity is
generally low to moderate (3 - 5 METs, metabolic equivalents) and
climbing stairs to the second floor is often used as equivalent
activity in terms of energy expenditure. Phosphodiesterase-5
inhibitors for the treatment of erectile dysfunction are usually
safe in CCS patients, but are contraindicated in those who take
nitrates and who have severe hypotension [86]. Healthcare
professionals should ask patients about sexual activity, give them
information and provide advice
Adhering to lifestyle modifications and taking medication regularly
is a big challenge. A systematic review of epidemiological studies
has shown that a significant proportion of patients do not adhere to
regular CV medications and that 9% of cardiovascular adverse events
in Europe can be attributed to poor patient adherence (compliance)
to regular therapy [87, 88]. In older men with CKD, greater
adherence to medication guidelines was positively associated with
better clinical outcomes, independent of other conditions.
Polypharmacy plays a negative role in adherence to treatment [88]
and the complexity of the medication regimen is associated with
non-adherence and a higher hospitalization rate [89]. Physicians who
prescribe drugs should give preference to drugs that have proven
their benefit with the highest level of evidence and those that are
most beneficial to the patient, without significant side effects of
the drug. Regime simplification helps adhere to treatment and there
is evidence of the benefits of cognitive education strategies,
electronically monitored feedback, and telephone support from nurses
and technicians. Reviewing and controlling the type and dose of
drugs by primary care physicians is a significant factor in helping
all patients, especially patients with more comorbidities, to
simplify the treatment regimen, detect drug interactions and
minimize the risk of drug side effects [89,90,91]. Long-term support
(intensive for the first 6 months, then every 6 months for 3 years)
in the GOSPEL study (Global Secondary Prevention Strategies to Limit
Recurrence after Myocardial Infarction) resulted in significant
improvements in risk factors and a reduction in some adverse
outcomes [20].
Sex hormone replacement therapy in menopausal women with CCS is not
recommended.
Annual vaccination against influenza is recommended for all patients
with CCS because it improves the prevention of AMI, reduces CV
mortality in adults aged> 65 years
PHARMACOLOGICAL THERAPY OF CHRONIC CORONARY SYNDROMES SCENARIO 1
PHARMACOLOGICAL THERAPY OF CHRONIC CORONARY SYNDROMES: CLINICAL
SCENARIO 1- Patients with suspected coronary heart disease and
stable angina and / or dyspnea on exertion.
The goals of pharmacological treatment of patients with CCS are: to
reduce the symptoms of angina and ischemia caused by physical
exertion and exercise and to prevent unwanted CV events.
Anti-ischemic drugs - but also lifestyle changes, regular exercise
training, patient education and eventual revascularization - all
play a role in minimizing or eradicating symptoms during long-term
prevention. Prevention of cardiovascular adverse events: ACS, AMI,
HFrEF, HFmEF, HFpEF), ventricular arrhythmias and heart blocks, VT,
AF, stroke and CV deaths associated with CCS focuses on reducing the
incidence of acute atherothrombotic events and and the development
of LV dysfunction. Optimal pharmacological therapy (OPhT)can be
defined as a treatment that satisfactorily controls symptoms and
prevents CAD-related adverse events, with maximum patient adherence
to treatment and with minimal drug side effects.
The modern role of first-line antianginal drugs: beta-blockers (BB)
and calcium antagonists (CCB) and second-line long-acting nitrates
(LANs) was highlighted, including new options: ivabradine,
nicorandil, trimetazidine and ranolazine (and possibly allopurinol),
and drugs that improve prognosis (acetylsalicylic acid (ASA) and
other antiplatelet drugs, statins, ACEI, BB). There are two
therapeutic goals in the treatment of CCS:
1. Improving the prognosis by reducing the risk of atherosclerosis
progression and preventing acute coronary events and sudden death
and prolonging life
2. Minimize symptoms with improved quality of life.
ANTI-ISCHEMIC (ANTIANGINAL) DRUGS
Immediate alleviation of anginal symptoms or prevention of symptoms
under circumstances that are likely to cause angina is usually
obtained by fast-acting formulations of nitroglycerin sublingually,
which is one of the first-line antianginal drugs. However, there is
no universal definition of optimal treatment in patients with HCV
and drug therapy must be tailored to the individual characteristics
and preferences of the patient. Initial drug therapy usually
consists of one or two antianginal drugs in addition to drugs for
the secondary prevention of CVD. [92-95]. The initial choice of
antianginal drugs depends on the expected tolerance associated with
the patient's profile and comorbidities, potential drug interactions
used concomitantly in therapy, patient preferences after
notification of potential adverse drug effects, and drug
availability. Combination therapy with two antianginal drugs e.g.
beta-blocker (BB) and calcium antagonist (CCB) are better than
monotherapy with any class of antianginal drugs, but the effect in
reducing clinical events remains unclear [95-98]. BB or CCB are
recommended as first-line drugs, although to date no randomized
controlled trial (RCT) has compared this strategy with alternative
strategies that use initial prescribing of other anti-ischemic drugs
or a combination of BB and CCB [92-95]. The results of a
meta-analysis of 46 studies and 71 treatment comparisons, support
the initial combination of BB and CCB. [98]. The same meta-analysis
suggested several other initial first-line combinations of
antiischemic drugs (long-acting nitrates, ranolazine, trimetazidine,
and to a lesser extent, ivabradine) that may prove useful in
combination with BB or CCB as first-line therapy, with no data for
nicorandil. No study or meta-analysis has yet sufficiently assessed
the impact of combining beta-blockers or CCBs with another line of
anti-ischemic drugs against adverse events: morbidity or mortality
[98]. Regardless of the initial strategy, the response to initial
antianginal therapy should be reconsidered 2 to 4 weeks after
starting treatment.
The algorithm of treatment with antiischemic drugs in patients with
suspected CCS and stable angina and / or dyspnea on exertion is
shown in TABLE 5.
TABLE 5 PHARMACOLOGICAL THERAPY OF CHRONIC
CORONARY SYNDROMES
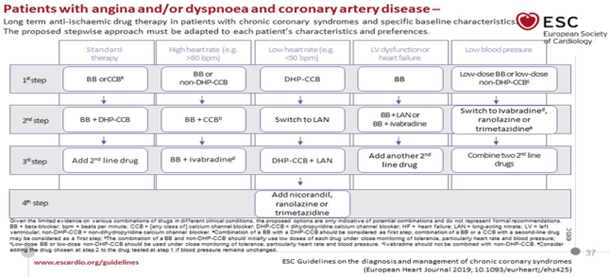
Retrieved from
https://www.escardio.org/Guidelines/Clinical-Practice-Guidelines/Chronic-Coronary-Syndromes
FIRST LINE TREATMENTS CCS
SHORT-ACTING NITRATES are given for an acute AP attack on exertion.
Sublingual tablets (lingvalete) and nitroglycerin spray provide
immediate relief of angina on exertion. The nitroglycerin spray
works faster than the sublingual nitroglycerin tablet [99]. At the
onset of angina symptoms, the patient should rest in a sitting
position (standing promotes syncope) and take nitroglycerin (tablet
0.3-0.6 mg sublingually, not swallowed, or 0.4 mg spray under the
tongue and not swallowed and not swallowed. inhale) every 5 min
until the pain ceases or a maximum of 1.2 mg is taken within 15 min.
Within that time frame, if the angina lasts for more than 15
minutes, the patient must call an ambulance for hospital treatment,
due to the suspicion of ACS. Nitroglycerin can be used for
prophylaxis before physical activities that are known to cause
angina
BETA BLOCKERS (BB-blockers of beta 1- β1-adrenergic receptors).
Selective β1-adrenergic receptor blockers are preferred in CCS. The
efficacy of OMT in stable angina where BB is the central component
of treatment is similar to the effect of percutaneous coronary
intervention (PCI) with a stent, according to W. Boden, principal
investigator of the COURAGE study [16, 60]. The dose of
beta-blockers should be adjusted so that the heart rate is 55-60
beats per minute [100, 101]. Discontinuation should be gradual dose
reduction and not abrupt. Abrupt cessation of intake due to an
increase in the number of β1 receptors in the heart causes worsening
of angina, sometimes even myocardial infarction. Dosing according to
the target heart rate of 55-60 / min is common, but is acceptable
below 50 / min in an individual patient without blocks. Target doses
of β1-blockers in CCS: metoprolol 2 x 100mg. maximum 400 mg,
bisoprolol 1 x 1.25-10 mg, maximum in Europe 30 mg, in the USA up to
40 mg; (L.Opie, Drugs for the Heart, 2013); nebivolol 1.25-5 mg x 1,
up to a maximum of 15 mg in practice. BBs are effective in silent
ischemia. During the effort, the goal is for the heart rate not to
be over 100 / min. All BBs are potentially equally effective in CCS
and selection is made according to comorbidities. BBs can be
combined with dihydropyridine (DHP) CCBs to reduce DHP-induced
tachycardia. Caution is warranted when a beta-blocker is combined
with verapamil or diltiazem due to the potential for the development
of worsening SI, excessive bradycardia, and / or atrioventricular
block (formerly an absolute, now a relative contraindication). The
combination of a beta blocker with nitrate reduces reflex
tachycardia. The main side effects of beta blockers are fatigue,
mental depression, bradycardia, AV block, bronchospasm, peripheral
vasoconstriction, postural hypotension, impotence, and masking the
symptoms of hypoglycemia. In comorbidities, the most selective β1
blockers bisoprolol and nebivolol are preferred. In patients with
recent AMI and those with chronic SI with reduced EF (HFrEF), BB is
associated with a significant reduction in mortality and
cardiovascular events [102–104], about a 30% reduction in mortality
and reinfarction, and a similar effect in ischemic SI. The benefit
in patients with CAD without previous AMI or SI is less well
established and placebo-controlled trials are lacking. [105]. A
retrospective analysis of 21860 matching patients from the REACH
registry did not show a reduction in cardiovascular mortality with
beta-blockers in patients with CAD and risk factors, with or without
previous AMI [106], but this is still the subject of debate and
further research.
CALCIUM ANTAGONISTS (CCBs or calcium channel blockers).
While CCB improves the symptoms of myocardial ischemia, it has not
been shown to reduce adverse events and mortality in patients with
CCS [107]. However, they have been shown to have an advantage in the
prevention of exercise ischemia over BB.
NON-DIHYDROPYRIDINE CALCIUM ANTAGONISTS (NE-DHP): VERAPAMIL AND
DILTIAZEM
Verapamil has a number of approved indications, including all types
of angina (on exertion, vasospastic and unstable), supraventricular
tachycardia and hypertension. Indirect evidence suggests good
safety, but with the risk of heart blocks, bradycardia and HF.
Compared with metoprolol, antianginal activity was similar. Combined
beta-blockade with verapamil is not recommended (previously
absolutely contraindicated due to the risk of cardiac SA and AV
blocks). Diltiazem, with its profile of effects, has advantages over
verapamil in the treatment of exertion angina. Like verapamil, it
acts by peripheral vasodilation, reducing afterload while preventing
coronary vasospasm. It has a moderate negative inotropic,
chronotropic and dromotropic effect. There were no test results
comparing diltiazem and verapamil. The use of non-DHP is not
recommended for CCB in patients with LV dysfunction
CALCIUM DIHYDROPYRIDINE ANTAGONISTS (DHP-CCB)
Long-acting nifedipine
Nifedipine, a potent arterial vasodilator, is particularly well
tested in hypertensive anginal patients when added with
beta-blockade. In large placebo-controlled ACTION, the addition of
long-acting nifedipine [60 mg once daily] to conventional angina
treatment had no effect on prognosis. Long-acting nifedipine has
been shown to be safe and has reduced the need for coronary
angiography and cardiovascular interventions [108]. Relative
contraindications for nifedipine are: small cardiac output (severe
aortic stenosis, hypertrophic obstructive cardiomyopathy or HF);
Careful combination with beta-blockade is usually feasible and
desirable. Vasodilator side effects include headaches and ankle
edema.
Amlodipine.
The very long half-life of amlodipine and its good tolerability make
it an effective antianginal and antihypertensive drug taken once a
day. There are few side effects, mostly ankle edema. In patients
with CCS and without SI, amlodipine at a dose of 10 mg / day reduced
the number of coronary revascularizations and hospitalization for AP
in a 24-month study [109]. Exercise-induced ischemia is reduced more
effectively with amlodipine, 10 mg / day, than with the beta-blocker
atenolol, 50 mg / day, and their combination is even better.
However, the CCB-BB combination is underused, as are other
combinations of antichemical drugs, even in some studies that report
that “optimal treatment of stable AP on exertion” has been applied
[110]
SECOND LINE DRUGS
Long-acting nitrates for the prophylaxis of angina (eg,
nitroglycerin patch, isosorbide dinitrate, and isosorbide
mononitrate) are second-line drugs for relieving AP, when initial
therapy with BB or NE-DHP CCB is contraindicated, poorly tolerated,
or insufficient to control symptoms. There is essentially a lack of
data comparing nitrates with BB and CCB, in order to draw firm
conclusions about their relative efficacy [110]. When taken over a
long period of time, long-acting nitrates cause tolerance with loss
of efficacy, so it is necessary to prescribe a drug-free interval of
10-14 hours. The bioavailability of isosorbide dinitrate depends on
inter-individual liver variability while isosorbide mononitrate, its
active metabolite, is 100% bioavailable. Dose titration is essential
to obtain maximum symptom control at a tolerable dose.
Discontinuation should be a gradual dose reduction and not abruptly
avoid worsening of AP. The most common side effects are hypotension,
headache and redness. Contraindications include hypertrophic
obstructive cardiomyopathy, severe aortic stenosis, and concomitant
use of phosphodiesterase inhibitors (e.g., sildenafil, tadalafil, or
vardenafil) or riociguata.
Molsidomin is an unfairly neglected drug (even in the new ESC guide
from 2019 [1]), which acts similarly to nitrates, but does not
develop tolerance to its action, has an effective anti-ischemic
effect and good tolerability. Dosage 3 x 2mg to 4mg or retractable
form 2 x 8mg. Unfortunately, there are no studies on the effect on
the prognosis of CCS [111,112]
Ivabradine is not inferior to atenolol or amlodipine in the
treatment of angina and ischemia in patients with CCS [111]. By
adding ivabradine 7.5 mg twice daily, atenolol therapy provided
better control of heart rate and anginal symptoms. Overall, the
results of the study support the use of ivabradine as a second-line
drug in patients with CCS, when they do not tolerate or have
contraindications for BB.
Nicorandil is a nitrate derivative of nicotinamide, with antianginal
effects similar to those of nitrates or beta blockers. Side effects
include nausea, vomiting, and potentially severe ulceration of the
oral, intestinal, and mucous membranes. In a placebo-controlled IONA
study (n = 5126), nicorandil significantly reduced nonfatal AMI or
hospitalization in patients with CCS, but there was no effect on
death from ischemic heart disease or fatal AMI [113]. These results
support the use of nicorandil as a second-line drug in patients with
CCS.
Ranolazine is a selective inhibitor of late internal sodium current.
Side effects include dizziness, nausea and constipation. In
addition, ranolazine increases QTc, and should therefore be used
with caution in patients with QTc prolongation or with QTc
prolonging drugs. In a placebo-controlled study in 6560 patients
with NSTEMI ACS, the addition of ranolazine to standard treatment
did not prove effective in reducing primary outcomes and CV
mortality, AMI, or recurrent ischemia. [114]. However, ranolazine in
the relatively large CCS subgroup (n = 3565) significantly reduced
recurrent ischemia and worsening angina [115]. These results support
the use of ranolazine as a second-line drug in patients with CCS
with angina despite frequently used antianginal agents such as
beta-blockers, CCB, and / or long-acting nitrates. In contrast,
there is a lack of evidence to support the use of ranolazine in
patients with CCS after PCI with incomplete revascularization
Trimetazidine reduces ischemia by affecting myocardial metabolism
without hemodynamic effects, unlike many anti-ischemic drugs [116].
Trimetazidine 35 mg twice daily BB (atenolol) reduces
exertion-induced ischemia [117]. It is contraindicated in
Parkinson's disease and movement disorders.
A study of 1628 patients showed that treatment with trimetazidine
along with other antianginal drugs resulted in a lower mean number
of mild angina attacks.
Allopurinol, a xanthine oxidase inhibitor, has recently been
proposed for the treatment of CCS. Allopurinol has a double effect
of energy conservation, reduces the consumption of O2 in the
myocardium by inhibiting xanthine oxidase and transfers from
creatine phosphate to ATP. Norman et al [118] in a randomized study
of 65 patients with CCS found that 600 mg / day of allopurinol
prolongs the time to onset of ST depression and pain by reducing
vascular oxidative stress. the development of acute myocardial
infarction (ACS) in the elderly, especially when taken for more than
2 years [119,]. However, the role of allopurinol in reducing
clinical events in CAD remains unclear [120].
PATIENTS WITH CCS AND LOW BLOOD PRESSURE
Therapy with anti-ischemic drugs should be started with very low
doses, BB or non-DHP-CCB with vigilant monitoring of tolerance to
these drugs, and in case of severe hypotension, therapy should be
discontinued. Preference should be given to drugs that do not affect
blood pressure, such as: Trimetazidine, Ranolazine and Ivabradine in
patients with sinus rhythm
PATIENTS WITH CCS AND BRADICARDIA
Elevated resting heart rate is a strong independent risk factor for
adverse events in patients with CCS and the therapeutic goal is a
heart rate (SF) of less than 60 / min. But with HR <50 / min, drugs
that have a negative chronotropic effect (BB and NON-DHP-CCB,
ivabradine) should be avoided or used with caution if necessary.
Treatment should begin with a very low dose. Drugs that do not have
a heart rate slowing effect should be preferred (DHP-CCB, LAN,
Trimetazidine, Ranolazine, Nicorandil)
PHARMACOLOGICAL TREATMENT TO IMPROVE PROGNOSIS AND PREVENT ADVERSE
EVENTS
ANTI-PLATELET MEDICINES
Platelet activation and aggregation is the initiator of symptomatic
coronary atherothrombosis, which is the basis for the use of
antiplatelet - antiplatelet drugs in patients with CCS, given the
favorable balance of prevention of ischemic events and increased
risk of bleeding. Dual antiplatelet therapy (DAPT) with aspirin and
oral P2Y12 inhibitors is the basis of antithrombotic therapy after
AMI and / or PCI.
ACETYLSALICYLIC ACID (ASPIRIN) IN SMALL DOSES acts by irreversibly
inhibiting platelet cyclooxygenase-1 and thus thromboxane, which
occurs with a chronic dosage of ≥75 mg / day. Gastrointestinal side
effects at higher doses justify a daily dose of 75-100 mg for the
prevention of ischemic events in CAD patients with or without a
history of AMI. As inhibition of cyclooxygenase-1 by aspirin is
consistent and predictable in adequate patients, there is no need to
test for platelet function.
P2Y12 INHIBITORS block platelet receptors P2Y12, which plays a key
role in platelet activation and arterial thrombus formation.
Clopidogrel and prasugrel are thienopyridine prodrugs that
irreversibly block P2I12 with active metabolites. Clopidogrel is a
well-known standard antiplatelet drug, but relatively often there is
resistance to its action. Prasugrel does not show significant
resistance, reduces ischemic events and stent thrombosis, but
without affecting mortality, but therefore to the detriment of
increased nonfatal bleeding. Ticagrelor is a reversibly binding
inhibitor of P2Y12, which does not require metabolic activation.
Ticagrelor has the most predictable and consistently high level of
P2Y12 inhibition during maintenance therapy in susceptible patients
and also has a faster onset of action compared to clopidogrel.
Ticagrelor monotherapy appears to have similar efficacy and safety
as aspirin in patients with previous PCI. Ticagrelor increases
non-fatal but not fatal bleeding. Equivalent efficacy and similar
safety of two doses of ticagrelor were explained by similar levels
of platelet inhibition. Ticagrelor can cause dyspnoea, which is
often transient and usually mild and tolerable, but sometimes a
switch to thienopyridine is required. There are opinions and limited
pharmacodynamic studies that support the unlicensed use of prasugrel
or ticagrelor in stable patients undergoing elective PCI who are at
high risk for stent thrombosis.
DURATION OF DOUBLE ANTIAGREGATION THERAPY AFTER PCI
After 6 months, DAPT achieves an optimal balance of efficacy and
safety in most patients [121]. Premature discontinuation of P2Y12
inhibitors is associated with an increased risk of stent thrombosis
and is not recommended [121]. However, a shorter duration of DAPT
may be considered in individuals at high risk for life-threatening
bleeding given the very low risk of stent thrombosis after 3 months.
However, the official position is: 12 months of DAPT recommended
after ACS and PCI.
Greater benefit from prolonged therapy with clopidogrel or prasugrel
has been observed in patients treated with AMI. The PEGASUS-TIMI 54
study showed that long-term therapy with ticagrelor 60 or 90 mg 2 x
1, initiated in stable patients more than 1 year after AMI, reduced
ischemic events at the expense of increased multiple nonfatal
bleeding. [121]. The 60 mg dose appears to be better tolerated and
has been approved in many countries for this indication. Absolute
reduction of ischemic events in CCS SCENARIO 4 with long-term
ticagrelor (60 mg 2 x 1) with a low dose of ASA in high-risk
patients after AMI with DM, peripheral arterial disease or
multivessel CAD was demonstrated by Bhatt DL and associates in the
subgroup of the mentioned study PEGASUS- TIMI 54. [122].
ORAL ANTICOAGULANT MEDICINES (AOK)
ANTICOAGULANT DRUGS IN SINUS RHYTHM
Anticoagulant drugs inhibit the action and / or production of
thrombin, which plays a key role in both coagulation and platelet
activation. Recently published studies have renewed interest in
combining lower anticoagulant doses with antiplatelet therapy.
RIVAROXABANE IN SMALL DOSES. Rivaroxaban is a factor Xa inhibitor
that has been studied at a low dose of 2.5 mg 2 x 1 daily in several
populations of patients with sinus rhythm, and this dose is 1/4 of
the standard dose used for anticoagulation in patients with AF. In
the ATLAS ACS 2TIMI 51 study, rivaroxaban 2.5 mg 2 x 1, compared
with placebo, reduced the complex outcome of AMI, stroke, or CV
death in stabilized patients treated with aspirin and clopidogrel
after ACS, with increased nonfatal bleeding, but with evidence
reductions in cardiovascular mortality [123]. Subsequently, in the
COMPAS study (124), the same regimen in combination with aspirin and
clopidogrel, with or without rivaroxaban 2 x 5 mg, in patients with
CCS showed reduced ischemic events at the expense of an increased
risk of predominantly nonfatal bleeding. [124].
ANTICOAGULANT DRUGS IN ATRIAL FIBRILLATION
OAC is recommended for patients with AF and CCS to reduce ischemic
stroke and other ischemic events. OAC in patients with AF has shown
superiority over aspirin monotherapy or clopidogrel-based DAPT for
stroke prevention and is therefore recommended for this indication.
[124]. When administering OAC to a patient with AF and CCS, based on
the CHA2DS2-VAS score and HASBLED score, non-vitamin K antagonists -NOAC
(i.e., apicaban, dabigatran, edoxaban, or rivaroxaban) have an
advantage over vitamin K (VKA) antagonists. [1]
Proton pump inhibitors
Proton pump inhibitors reduce the risk of gastrointestinal bleeding
in patients treated with antiplatelet drugs and are given to anyone
with a high risk of bleeding (HASBLED score) and monotherapy, to
improve safety. [124]
STATINS
When target LDL cholesterol values cannot be achieved, it has been
shown that the addition of ezetimibe reduces LDL cholesterol but
also reduces CV events in patients with ACS, in diabetics [1]
without further impact on mortality. In addition to exercise, diet,
and weight control, which should be recommended to all patients,
dietary supplements, including phytosterols, may lower LDL-C to a
lesser extent, but no improvement in clinical outcomes has been
shown [1]. Phytosterols are also used in patients with statin
intolerance, which is a group with a higher risk of cardiovascular
events. Studies from 2015 show that subtilinin-kexin type 9
proprotein convertase inhibitors (PCSK9) (evolocumab and alirocumab)
are very effective in lowering cholesterol, lowering LDL-C in a
consistently stable manner to <-1.3 mmol / L. In outcome studies,
these agents have been shown to reduce cardiovascular and mostly
ischemic events, with little or no effect on mortality. [1] Very low
cholesterol is well tolerated and associated with fewer events, but
the high cost of PCSK9 inhibitors and their unknown long-term safety
has limited their low-density lipoprotein apheresis and new
therapies such as mipomersen and lomitapid need further
investigation. For patients undergoing PCI, a high dose of
atorvastatin reduces the incidence of periprocedural events [1].
RENIN ANGIOTENSIN ALDOSTERONE SYSTEM BLOCKERS
ACE INHIBITORS can reduce mortality, AMI, stroke, and HF among
patients with LV dysfunction, previous peripheral vascular disease,
and high-risk DM. It is recommended that ACE inhibitors (or ARBs,
angiotensin AT2 receptor blockers in cases of ACEI intolerance) be
considered for the treatment of patients with CCS with coexisting
hypertension, LVEF <40%, DM or chronic renal disease and
insufficiency (CKD), unless contraindicated (eg severe renal
impairment, hyperkalaemia, etc.). However, not all studies have
shown that ACE inhibitors reduce all-cause mortality, as well as
cardiovascular death, nonfatal AMI, stroke, or HF in patients with
atherosclerosis and without impaired LC function. A meta-analysis,
including 24 trials and 61961 patients, documented in CCS patients
without HF [1] that renin-angiotensin system (RAS) inhibitors
reduced cardiovascular events and death only compared to placebo,
but not when compared to active controls. . Therefore, ACE inhibitor
therapy in CCS patients without HF or high CV risk is generally not
recommended unless necessary to achieve target blood pressure
values.
Neprilysin is an endogenous enzyme that degrades vasoactive peptides
such as bradykinin and natriuretic peptides. Pharmacological
inhibition of neprilysin raises the level of these peptides,
enhancing diuresis, natriuresis, myocardial relaxation, and
antiremodeling and reducing renin and aldosterone secretion. The
first drug in the class is LCZ696, which combines valsartan and
sacubitril (a neprilysin inhibitor) in one tablet. patients with HF
(LVEF <_35%) who remain symptomatic despite optimal treatment with
ACE inhibitor, beta blocker and mineralocorticoid receptor
antagonist (MRA), sacubitril / valsartan is recommended as a
replacement for ACE inhibitor to further reduce the risk of HF
hospitalization and death in outpatients.337 Aldosterone blockade
with spironolactone or eplerenone is recommended for use in post-MI
patients who are already receiving therapeutic doses of ACE
inhibitors and beta blockers and have LVEF <35%, or diabetes or SI-HF.
Caution should be exercised when using MRA in patients with impaired
renal function [estimated GFR (eGFR) <45 mL / min / 1.73 m2] and in
those with serum potassium> _5.0 mmol / L.
The combination of anti-ischemic drugs and drugs that affect the
prognosis and survival in practice are insufficiently used both in
the world [110] and in the part of Serbia - Timok region. Doses are
not titrated to achieve optimal effects of pharmacological therapy
[112]. The analysis of the treatment of DE NOVO coronary heart
disease in consecutive 101 pts is presented from the clinical
practice of the Dr. Bastać Practice. At the first examination,
patients, previously treated or understood as stable angina
pectoris, had pharmacological therapy by the attending physician or
cardiologist (only 26% had a definitive diagnosis of coronary heart
disease by exercise ECG test) attached to TABLE 6.
Table 6. Analysis of prescribed drugs for the
treatment of suspected chronic coronary syndrome (CCS) in the
Practice "Dr Bastać" on the 101st consecutive patient in 2017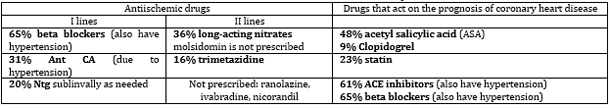
MYOCARDIAL REVASCULARIZATION (MR). The role of coronary
myocardial revascularization (MR) in the treatment of chronic
coronary syndromes SCENARIO 1. Figure 5
FIGURE 5. The role of coronary myocardial
revascularization (RM) in the treatment of chronic coronary
syndromes SCENARIO 1
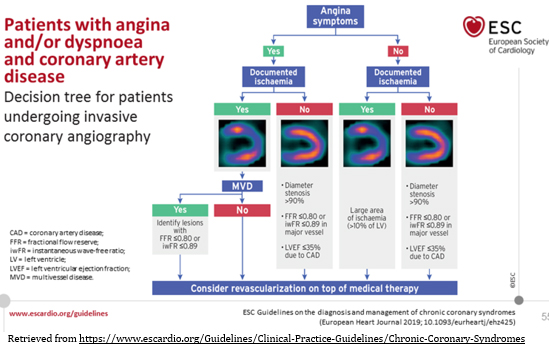
Retrieved from
https://www.escardio.org/Guidelines/Clinical-Practice-Guidelines/Chronic-Coronary-Syndromes
In patients with CCS, optimal medical therapy (lifestyle change,
risk factor reduction and pharmacological-drug therapy, do not
equate medical and drug therapy) -OMT is key to reducing symptoms,
stopping the progression of atherosclerosis and preventing
atherothrombotic events. Myocardial revascularization (RM) plays a
central role in the management of the most severe forms of CCS as a
last resort, but always as an adjunct to optimal medical therapy (OMT),
without its elimination. The goals of MR are to alleviate symptoms
in patients with angina and / or to improve the prognosis. These
recommendations suggest that revascularization in patients with AP
and significant stenosis is often second-line therapy when OMT has
not been successful. Myocardial revascularization: PCI or CABG can
effectively alleviate angina, reduce the use of antianginal drugs,
and improve exercise ability and quality of life in a small number
of selected patients compared to the OMT-only strategy.
Revascularization with either PCI or CABG also aims to effectively
eliminate myocardial ischemia and its adverse clinical
manifestations among patients with significant coronary stenosis and
to reduce the risk of major acute adverse CV events including AMI
and cardiovascular death.
Numerous meta-analyzes comparing MR strategy by PCI with initial OMT
in patients with CCS show either no benefit [126, 127] or myocardial
revascularization provides a modest benefit [128, 129,] in terms of
survival or lower incidence of AMI and SI. In this regard, previous
ESC guidelines from 2013 [4] identified specific subgroups of
patients (based on coronary anatomy, size of myocardial ischemia
zone, risk factors, cardiac status, etc.) in whom MR may improve
prognosis, indicating that in other subgroups it has no effect.
FIGURE 5 summarizes the practical approach to MR indications in CCS
according to the presence or absence of symptoms and documented
myocardial ischemia by noninvasive functional imaging tests.
However, the risk-benefit relationship in an individual case should
always be evaluated and the MR is considered only if the expected
benefit outweighs the potential risk. Also, the aspect of joint team
decision-making is crucial, with complete information given to the
patient about the expected advantages and disadvantages of the two
strategies, including the risk of bleeding associated with DAPT in
cases of revascularization via PCI.
A detailed discussion of the best choice between PCI or CABG
revascularization modalities for an individual patient on the HEART
team was published in the 2018 ESC guidelines for myocardial
revascularization [131].
The role of MR has been placed in the context of recent evidence
relating to the prognostic role of percutaneous coronary
interventions (PCI) or coronary artery bypass grafting or native a.
mammario internal and other arteries (CABG) in this low-risk
population. MR is now reserved for patients where there is strong
evidence to improve prognosis based on evidence of regional ischemia
by visual noninvasive tests - perfusion imaging or assessment of FFR
and iwFR [131]. The typical constellation is in a patient with a
large area of myocardial ischemia corresponding to left main
stenosis (left main stenosis> 50%) and multivessel disease that
always involves stenosis ≥70% of the proximal anterior-descending
branch of the left coronary artery (LAD). There is unequivocal
evidence that percutaneous coronary revascularization (PCI) in acute
coronary syndromes with ST-segment elevation reduces mortality
relative to fibrinolysis, and both relative to those where no
reperfusion has been performed.
In other forms of CAD -chronic coronary syndromes (CCS), the role of
PCI revascularization is controversial in terms of mortality
reduction [132, 133]. A recent meta-analysis of 46 studies in 37,757
individuals examined the PCI benefit of various categories of
coronary patients, including true stable angina without a recent
heart attack. In stable angina pectoris, the chronic coronary
syndrome PCI categories did not reduce overall mortality (RR, 0.98,
p = 0.11]), cardiac death (RR, 0.89, p = 0.33), or myocardial
infarction (RR, 0.96; P = 0.54). PCI prevents death, cardiac death,
and AMI primarily in patients with unstable AP. For patients with
stable CAD, PCI shows no effects on any of these outcomes.
[132-134]. However, it is now becoming evident that OMT is still
underused in recent studies [132]. Mohee K. et al show that OMT is
still suboptimal in patients before PCI and becomes optimal only
after PCI due to increased compliance. [132]. Argument: OMT is the
definitive therapy for patients with stable coronary heart disease
and low risk of CV events (mortality <1% per year).
COURAGE STUDIES [16,60,133,134]. (Boden WE et al., Published in NEJM
2007) - initial PCI with a stent does not reduce the risk of death,
AMI and hospitalization and has no advantage in stable AP according
to OMT on 2287 randomized pts with known significant stable CAD and
proven myocardial ischemia who were only on OMT OR OMT + PCI.
Between 1999 and 2004, a COURAGE (Clinical Outcomes Utilization
Revascularization and Aggressive Drug Evaluation) study randomized
2287 pts with objective evidence of ischemia and proximal
angiographic CAD (≥ 70% visual visual stenosis) to OMT with or
without PCI. The aim and design of the study was to test the
strategy of routine, anatomically-indicated PCI, if necessary, for
the failure of the initial OMT. Follow-up of 2.5 to 7 years (median
4.6 g) showed that death or AMI occurred with the same frequency in
both subgroups ( PCI + OMT vs OMT = 1.05, p = 0.62). After 4.6 years
of follow-up, there was no statistically significant difference
between the groups for cumulative mortality and nonfatal myocardial
infarction -18.5% vs 19%, as well as for stroke and hospitalization
due to new unstable AP. Important: COURAGE study patients had marked
symptoms at enrollment and had significant comorbidities, a high
prevalence of objectively established ischemia, and extensive
angiographic coronary heart disease, and were in the population
where clinical benefit from PCI was expected.
Subgroup analysis reveals consistency among clinically relevant
subgroups. There is no difference only OMT versus OMT + PCI in terms
of multidisciplinary disease, low EF, class III-IV angina and the
presence of Diabetes Mellitus. By comparison, there was no
difference in hospitalization for ACS either. The main result of the
study showed that PCI as an initial strategy in patients with stable
AP CCS does not reduce death, AMI and other major events (MACE) when
OMT is added. Patients with PCI had less angina in the first and 3rd
year, but not in the 5th year of follow-up. As initially expected,
revascularization was more common in OMT in 16.5% of the first year
of follow-up alone. The efficacy of OMT in stable AP where the
optimal dose of beta-blocker is a central component is similar to
the effect of percutaneous coronary intervention (PCI) with a stent
(Boden and Courage, 2007). [133,134].
Comparison between PCI and OMT (Braunwald s Heart disease, 2015-
Morow D, Boden WE) [135]. n-) in terms of earlier techniques balloon
angioplasty vs medical therapy, belongs to history, now in the era
of new PCI techniques and new optimal drug therapy. In 16 studies of
about 9000 pts, PCI vs OMT, the invasive strategy did not provide a
reduction in mortality or AMI, but only a reduction in AP severity
and a better quality of life- QoL.
META-ANALYSIS OF VINDECKER et al. Reports reduction in death and AMI
by revascularization versus OMT only in patients with CCS when CABG
revascularization or next-generation drug-coated stent (DES) was
performed, as opposed to balloon angioplasty, metal BMS stents, and
old earlier DES [129].
FAME 2 [130]: Statistically significant risk reduction with PCI +
OMT versus OMT alone, discontinued after 7 months, but had
significant limitations, was not randomized, and was not a
double-blind controlled study. Nevertheless, Xaplanteris P. I et al.
Indicate a potentially broader prognostic impact of the
revascularization strategy when targeted with a functional invasive
assessment of coronary stenosis via FFR or iwFR. A five-year
follow-up of the FAME 2 study confirmed clinical benefit in a subset
of patients specifically treated with PCI targeting only
ischemia-producing stenoses (i.e., FFR <0.80) plus OMT, which
yielded significantly lower rates of emergency revascularization and
lower rates of spontaneous AMI [130] but without a clear effect on
mortality.
ORBITA A new ORBITA study (a randomized, controlled, double-blind
study) comparing OMT or angioplasty with an anatomically significant
coronary stenosis (PCI) stent in stable angina, with a false
invasive procedure (shame) in the control group, found no advantage
of PCI in significantly improving functional capacity. [125]. The
study highlights a significant placebo component on clinical effects
and warns us of the pitfalls of interpreting end-points that are
subject to bias in the absence of false control. However, the
results of the ORBITA study cannot provide definitive guidance due
to the limited size of the study, the short observation time to
treatment crossover, and insufficient strength to assess clinical
outcomes.
ISCHEMIA The largest international randomized double-blind
controlled follow-up study ISCHEMIA [137-139] recruited patients
with stable CAD with moderate or severe ischemia on a stress test
and aimed to assess whether there were differences in clinical
outcomes - mortality and CV morbidity in patients. with stable
chronic coronary heart disease (SCAD) between the invasive strategy
+ OMT and OMT alone. Out of a total of 8518 recruited patients, 5179
were randomly selected by randomization and were still randomized by
type of treatment: Invasive PCI with stent plus medical therapy (n =
2588) versus only medical therapy (n = 2591). Coronary CT
angiography was performed in most participants and was examined by
the baseline laboratory to rule out main stable stenosis ≥ 50%.
Randomized participants had a mean age of 64 years, with 1168 women
(22.6%) and 2122 diabetes (41.0%)). Among the 3909 participants
randomized after the functional imaging stress test for ischemia,
the assessment of the severity of ischemia in 3901 participants was
as follows: severe 1748 (44.8%), moderate 1600 (41.0%) and mild 317
(8.1%); 79.0% had multivessel CAD (n = 2679 of 3390) and anterior
descending branch (LAD) proximal stenosis 46.8%. During an average
of 3.3 years of follow-up, there was no significant statistical
difference between primary outcomes: death from cardiovascular
causes, myocardial infarction, or hospitalization for unstable
angina, cardiac arrest -13.3% in the invasive strategy versus 15.5%
in the medical treatment group. (p = 0.34). The key secondary
outcome was death from cardiovascular causes or myocardial
infarction: 11.7% in the routine invasive group versus 13.9% of the
OMT group (p = 0.21). Total mortality (cardiovascular and all other
causes): 6.4% in the routine invasive group versus 6.5% in the OMT
group (p = 0.67). Periprocedural AMI: 2.98%. The invasive versus
conservative relationship to mortality was similar regardless of the
degree of ischemia (p value for interaction = 0.23), which is also
true for AMI. (p value for interaction = 0.15). Among patients with
stable CAD and moderate to severe ischemia on a noninvasive
visualization stress test, routine invasive treatment showed a
reduction in major adverse outcomes compared with OMT. There is no
benefit from invasive PCI therapy in terms of a complex end result:
Overall, CV mortality, and nonfatal myocardial infarction.
The ACC / AHA American Guide to Chronic CAD [140] discourages the
use of PCI or CABG for single-vessel or double-vessel CAD without
significant proximal LAD involvement in the absence of unacceptable
AP after adequate guidance-guided OMT, especially if noninvasive
tests show a small area of exacerbated viable ischemia or reduced EF.
CLINICAL SCENARIO 2.
Patients with new-onset heart failure or left ventricular
dysfunction and suspected coronary artery disease
CAD is the most common cause of chronic heart failure (HSI) in
Europe. Most studies provide evidence to support recommendations for
the study of pts with ischemic cardiomyopathy (CMP) - the
pathophysiological basis of ischemic CMP is systolic dysfunction EF
<40%, although patients with CCS may also have HSI with preserved EF.
Patients with symptomatic HF should be diagnosed according to the
2016 ESC HF Guide [141]. In addition to standard medical history,
physical examination, ECG and chest radiography, Doppler
echocardiography should be included in the image to evaluate the
evidence of diagnosis of ischemic cardiomyopathy with HF with: a)
reduced EF; b) mid-range EF; C) preserved EF with focal or diffuse
echocardiographic signs of left and / or right ventricular systolic
dysfunction, evidence of diastolic dysfunction, compensatory
hypertrophy, valve function (ischemic mitral regurgitation) and
evidence of secondary pulmonary hypertension [1]. In addition to
routine hematological and biochemical analyzes, it is especially
important to assess renal function, potassium, and sodium initially
and during pharmacotherapy titration.
Measurement of serum natriuretic peptide levels serves to rule out
HF if NT-proBNP levels are normal. The degree of increase in GNP
titer is used to estimate the severity of HF [142]. The basic
treatment in the NYHA II-III class is antianginal or antischemic
therapy with drugs that affect the prognosis and prevention of
events. BBs are also an essential component in the relief of anginal
attacks and event prevention as well as mortality reduction in HF
(Class I level A) [142-149]. Amlodipine may be included as an
anti-ischemic drug, in those who do not tolerate BB I the only
calcium antagonist is considered safe in HIS (Class IIb level B
recommendation) [150-151]. Short-acting nitrates by sublingual or
transcutaneous patches are also recommended. Patients with
symptomatic HF should be treated according to the 2016 ESC Guide to
HF [141], with an emphasis on adding to the standard therapy the
newer drug angiotensin receptor-neprilysin inhibitor (sacubitril-valsartan)
[141]. Doses of drugs: diuretics [152], ACEI, BB, spironolactone or
eplerenone possibly and ivabradine should be gradually increased to
avoid hypotension, bradycardia, azotemia and hyperkalemia. ACEIs are
crucial (CLASS I, LEVEL A) in asymptomatic left ventricular
dysfunction after myocardial infarction and in symptomatic HF for
symptom relief and reduction of morbidity and mortality [153].
Implantable cardioverter-defibrillator (ICD) and cardiac
resynchronization therapy (CRT) [154] can provide improvement in
symptoms and improve survival [154]. Myocardial revascularization
should be considered in suitable patients with ischemic HF based on
symptoms, coronary anatomy, and evidence of current ischemia and
myocardial viability by imaging tests in akinesia-cicatricial zones,
through a multidisciplinary team. Myocardial revascularization is
recommended when anginal discomfort and / or dyspnoea persist
despite optimal antiaginal therapy (CLASS I, level of evidence A).
THE GROUP OF PATIENTS WITH LONG-TERM Dg CHRONIC CORONARY SYNDROMES
INCLUDE:
CLINICAL SCENARIO 3. ASYMPTOMATIC AND SYMPTOMATIC PATIENTS WITH
STABILIZED SYMPTOMS UP TO 1 YEAR LAST AFTER ACS OR CORONARY
REVASCULARIZATION.
CLINICAL SCENARIO 4: THE SAME ONLY AFTER A YEAR)
Common to both groups, clinical scenarios 3 and 4 are lifelong
treatment and follow-up [1]. The clinical course may be favorable
for a longer period. However, patients with CCS may develop various
CV complications: episode de novo ACS, complications due to
therapeutic procedures due to CAD or due to interactions with
comorbidities. The risk of complications also exists in asymptomatic
patients, so the risk assessment must be applied to both symptomatic
and asymptomatic patients. Therefore, a risk based on biomarkers in
2017 was developed and validated [155].
CLINICAL SCENARIO 3
ASYMPTOMATIC AND SYMPTOMATIC PATIENTS WITHIN 1 YEAR OF ACS OR
REVASCULARIZATION
After revascularization or stabilization of ACS within one year, the
patient must be closely monitored due to the increased risk of
complications and the need to adjust pharmacological treatment [35].
It is recommended to have at least two visits to the doctor in the
first year of follow-up, while those with systolic LC dysfunction
before revascularization or after ACS should report for cardiac
examination 8-12 weeks after ACS or revascularization. Cardiac
function can be improved by recovering from stunning or hibernating
myocardium by revascularization based on the results of the ADVISE
II study by invasively assessing whether epicardial coronary
stenosis is the cause of ischemia by iwFR [52,53]. However,
exacerbations can occur due to concomitant CV disorders (valvular
diseases, infections, arrhythmias, etc.) and these disorders need to
be identified and treated. Non-invasive post-revascularization
assessment may be considered to rule out residual ischemia or to
document a reference ischemia finding to plan treatment
intensification and further periodic follow-up [1].
CLINICAL SCENARIO 4.
ASYMPTOMATIC AND SYMPTOMATIC PATIENTS MORE THAN 1 YEAR LAST OF ACS
OR REVASCULARIZATION.
Once a year, the patient needs to be evaluated by a cardiologist,
even when the patient is without problems. The recommendation is an
annual clinical examination and assessment of adherence to
pharmacotherapy and non-pharmacological measures with the
determination of risk profiles through risk scores. Laboratory
analyzes: lipid profile, renal function, blood count and cardiac
biomarkers should be performed every two years [1, 45]. An elevated
marker of hsCRP inflammation is associated with an increased risk of
adverse events. Von Willebrandt factor, interleukin-6 and NTpro BNP
are predictors of outcome [25]. Other biomarkers for pts with CCS
also have prognostic significance: heart rate, hemoglobin, leukocyte
count [156]. Multiple biomarker scores showed prognostic
significance: combining: hsCRP, fibrin degrading products, and heat
shock protein 70; [157]. For patients with an exacerbated risk score
during follow-up, it is warranted to intensify therapy and
diagnostic re-evaluation, although risk-guided therapy has not yet
been shown to improve prognosis. A 12-channel ECG should be part of
each follow-up to record heart rate and rhythm and to detect changes
suggestive of symptomatic myocardial ischemia / infarction and to
evaluate PR, QRS, and QT intervals. It would be useful to
echocardiographically assess systolic and diastolic LV function,
valve status, heart size, and volume in apparently (seemingly)
asymptomatic patients at 3 to 5 years of age. [1,52,53]. In case of
unexplained reduction of left ventricular systolic function,
especially regional, it would be useful to do an image of coronary
anatomy. Asymptomatic, silent ischemia should also be sought in
seemingly asymptomatic patients through periodic stress imaging
tests [1, 52].
CLINICAL SCENARIO 5.
PATIENTS WITH ANGINA PEKTORIS AND SUSPECT VASOPASTIC OR
MICROVASCULAR DISEASE.
Angina without obstructive disease in the epicardial coronary
arteries (INOCA). In clinical practice, there is a discrepancy
between the findings concerning coronary anatomy, the presence of
symptoms and the results of non-invasive tests often occur [13].
These patients deserve attention because APs with nonobstructive CAD
are associated with an increased risk of adverse clinical events
[14,15]. The low diagnostic contribution of ICA can be explained by
the presence of: (1) mild stenosis or diffuse coronary narrowing,
with underestimated functional significance; (2) microcirculation
disorders; (3) dynamic epicardial vessel stenosis caused by coronary
spasm or intramyocardial bridges that are not apparent during CTA or
ICA. Intracoronary pressure measurements are useful in resolving the
first case. At diagnostic processing, patients with angina and / or
myocardial ischemia who have coronary stenoses with nonischemic FFR
or ivFR values may also be referred to as non-obstructive diseases
of the epicardial coronary arteries. The presence of clearly defined
anginal symptoms and pathological findings of non-invasive tests in
patients with normal coronary epicardial arteries should lead to
suspicion of a non-obstructive cause of ischemia. Often and mainly
as a result of persistence of symptoms, patients with angina and
without obstructive CAD undergo multiple diagnostic tests, including
repeated coronary CTA or ICA, which contribute to increased health
care costs [158]. It is important to emphasize that in everyday
practice, there is often a significant discrepancy between the
findings of coronary anatomy, the presence of symptoms, and the
results of noninvasive tests. Diagnostic pathways investigating
microcirculatory or vasomotor coronary disorders are often not
performed, so a definitive, evidence-based diagnosis is rarely made.
Due to that, patient anxiety and depression are not uncommon in this
clinical population. [159]. A 2018 randomized controlled trial of
CorMicA found in patients with nonobstructive coronary heart
disease, through customized treatment guided by the results of an
intracoronary trial: coronary flow reserve (CFR), microcirculatory
resistance, and acetylcholine test resulted in a significant
reduction in anginal symptoms compared with treatment [160].
Microvascular angina
Patients with microvascular angina typically have chest pain on
exertion, a positive ischemia test, either exercise ECG test or
noninvasive imaging tests, without obstructive CAD or with mild to
moderate stenosis (40-60%) of the epicardial coronary arteries,
which is detected by IC CTA and these stenoses are considered
functionally insignificant. The microvascular origin of angina is
usually suspected after the exclusion of obstructive coronary
epicardial stenosis, during the diagnostic processing of patients
with proven myocardial ischemia on the exercise ECG test. Regional
abnormalities of wall movement rarely develop during exercise or
stress in patients with microvascular angina. Some patients may have
a mixed form of microvascular + vasospotic angina, with occasional
episodes at rest, especially associated with exposure to cold.
Secondary microvascular angina, without obstructive CAD, may be due
to cardiac or systemic conditions, including those that cause LV
hypertrophy (such as hypertrophic cardiomyopathy, aortic stenosis,
and hypertensive heart disease) [161] or inflammation (such as
myocarditis or vasculitis). ) [162]. Risk stratification in
microvascular AP is quite complex. The presence of microcirculatory
dysfunction in patients with CCS entails a worse prognosis than
originally thought, based on the latest evidence based on monitoring
patients with objective microcirculation disorders proven by
invasive or noninvasive techniques. [163-167]. Microcirculation
dysfunction precedes the development of epicardial coronary lesions,
especially in women, and is associated with impairment and adverse
outcome events. Among diabetic patients undergoing diagnostic
processing, those without obstructive epicardial disease but with
abnormal coronary flow reserve (CFR) have a similarly poor long-term
prognosis as those with obstructive epicardial disease [165]. In
patients with significant CAD with significant stenosis at FFR ≤
0.80, the presence of abnormal CFR <2.0 is associated with
additional exacerbation and a significant number of adverse events
especially when the microcirculatory resistance index (IMR) is also
abnormal [166].
The possibility of microcirculatory origin of angina should be
considered in patients with clear angina, abnormal non-invasive
functional tests, and coronary arteries that are either normal or
with mild stenosis, which are considered functionally insignificant
on ICA or CTA. One of the challenges in performing a comprehensive
assessment of microvascular function is to test the two main
mechanisms of dysfunction separately:
1. Weakened microcirculatory conductivity (or increased
microcirculatory resistance)
and
2. Arteriolar dysregulation. [168-170].
However, it should be clarified which of these two pathways is
critical to the choice of pharmacological treatment to minimize the
symptoms of these patients. [160]. Weakened or disturbed
microcirculatory conduction can be diagnosed by measuring coronary
flow reserve (CFR) or minimal microcirculatory resistance. CFR can
be measured by noninvasive transthoracic color and pulsed Doppler
echocardiography [visualization and measurement of basal flow rate
and with vasodilatation test with adenosine or dipyridamole] [171]
as well as magnetic resonance imaging (myocardial perfusion index is
less available or PET). Microcirculatory resistance can be measured
in a catheterization laboratory by combining intracoronary pressure
with thermodilution-based data (to calculate IMR) or Doppler flow
rates (to calculate hyperemic microvascular resistance or HMR)
[172,173]. The decision on abnormal microcirculation is made when
the value of the microcirculatory resistance index is greater than
25 units (IMR> 25 J) or CFR <2.0. In contrast, the diagnosis of
arteriolar dysregulation requires assessment of endothelial function
in coronary microcirculation by selective intracoronary infusion of
acetylcholine. Acetylcholine is an endothelium-dependent vasodilator
that acts directly on arteriole smooth muscle cells and causes
paradoxical arteriolar vasoconstriction — microvascular spasm of
dysfunctional vascular endothelium or abnormal smooth muscle cell
function [174]. This arteriolar response to acetylcholine causes
anginal symptoms with or without concomitant ischemic ECG changes
and a decrease in coronary blood flow velocities if simultaneous
Doppler measurements are performed. Peripheral pulse tonometry
during reactive hyperemia may also reveal abnormal systemic
endothelial function in patients with AP and non-obstructive CAD
[175].
Vasospastic angina
Vasospastic angina should be suspected in patients with AP when the
symptoms occur mainly at rest, with maintained tolerance to
exertion. The likelihood of vasospastic angina increases when the
attacks follow a circadian pattern, with multiple episodes at night
and in the early morning hours. Patients are often younger and have
fewer CV risk factors than patients who have AP on exertion, except
that they are most often cigarette smokers [176]. Coronary vasospasm
is also suspected in patients with transient coronary stents and
persistent AP. [177-178]. The diagnosis of vasospastic AP is based
on the detection of transient ischemic changes in depression or
elevation of the ST segment during an angina attack (usually at rest
rest AP). Patients with Prinzmetal's AP represent a special subgroup
where resting AP is accompanied by transient ST-segment elevation
[176-179]. This ST elevation on the ECG correlates with proximal
occlusion by vasospasm. As most vasospastic AP attacks are
self-limiting, it is difficult to register without multi-day
ambulatory Holter monitoring by 12-channel recording. The appearance
of ST-segment changes at normal pulse supports the probability of
myocardial ischemia caused by spasm. In patients with suspected
vasospastic AP and documented ECG changes, coronary CTA or ICA is
important to rule out the presence of fixed coronary stenosis.
Angiographic documentation of coronary spasm requires the use of a
provocation test in a catheterization laboratory. Due to the low
sensitivity of the hyperventilation test and the cold water test,
intracoronary administration of acetylcholine or ergonovine during
ICA are preferred provocative tests. [176-179]. 176. Both
pharmacological agents are safe, provided that they are selectively
inserted into the left or right coronary arteries and that activated
spasm is easily controlled by intracoronary nitrates. A small
percentage of patients may develop ventricular tachycardia /
ventricular fibrillation or bradyarrhythmia during a provocative
test (3.2 and 2.7%, respectively), similar to that during
spontaneous spasm attacks (7%) [180]. Intravenous administration of
ergonovine for non-invasive tests is contraindicated due to the risk
of causing long-term spasm in multiple coronary arteries, which can
be very difficult to stop and can be fatal.
A provocative test for coronary spasm is considered positive when it
causes: (1) anginal symptoms, (2) ischemic ECG changes, and (3)
severe vasoconstriction of the epicardial coronary artery. If the
test fails to run all three components, this should be considered
ambiguous [176]. The development of AP in response to acetylcholine
injections in the absence of angiographically evident spasm, with or
without concomitant ECG changes in the ST segment, may indicate
microvascular spasm and is often seen in patients with microvascular
AP [179]. In patients with epicardial or microcirculatory vasomotor
disorders, CCB and long-acting nitrates (LANs) are the treatment of
choice, in addition to controlling CV risk factors and lifestyle
changes. Nifedipine has been shown to be effective in reducing
coronary spasm associated with stent implantation. In all patients
with vasospastic angina, optimal control of risk factors should be
achieved, especially smoking cessation and aspirin use. Exclude
drugs that may be narrow vasospasm - cocaine or amphetamine abuse.
Chronic preventive treatment of vasospastic angina is mainly based
on higher doses of calcium antagonists. Average doses of these drugs
verapamil or diltiazem from 240 to 360 mg / day or nifedipine from
40 to 60 mg usually prevent spasm in about 90% of patients.
Sometimes high doses of calcium antagonists must be given to prevent
spasms: up to 960 mg a day of Verapamil or Diltiazem or up to 100 mg
/ day of Nifedipine. Long-acting nitrates should be added to some
patients. Beta-blockers should be avoided, as they may increase
spasm because by blocking the beta-dilating effects,
vasoconstriction by unblocked alpha receptors predominates. About
10% of patients are refractory to this treatment, so the addition of
guanethidine or clonidine may rarely be indicated. Stent
implantation at the site of spasm and without stenosis, as well as
surgical or chemical sympathectomy, are extreme measures.
CLINICAL SCENARIO 6. ASYMPTOMATIC PERSONS IN WHICH CAD WAS DETECTED
AT SCREENING.
In an effort to reduce the high incidence of coronary sudden death
in asymptomatic adults, numerous studies of risk factors and risk
indicators, as well as stress tests, are often performed as
screening tests. The European guidelines for CVD prevention in 2016
in clinical practice focused in detail on these issues. [15]. In
general, the use of a risk assessment system such as ESC SCORE is
recommended. People with a family history of premature CAD should be
screened for familial hypercholesterolemia. Coronary calcium score,
ABI index, and Color Doppler echosonography of the carotid arteries
in plaque detection may provide useful information on
atherosclerotic risk in selected patients. Routine use of biomarkers
or imaging tests for CAD is not recommended. New biomarkers have an
increasing predictive value compared to classical ones, but the net
improvement in reclassification risk is still only modest (7.18%)
compared, for example, with the coronary calcium score, which has a
net improvement in reclassification of 66%. Only persons at high
risk of events should be considered for further non-invasive or
invasive examination. There are no data on how to treat asymptomatic
subjects with a positive CAD test outside the recommendations
outlined in these guidelines. However, the principles of risk
stratification, as described above for symptomatic patients, also
apply to these individuals. It is important to know that there is no
data that showed an improved prognosis after appropriate care based
on new biomarkers. It is important to note that cancer patients and
those undergoing treatment for cancer, chronic inflammatory and
systemic autoimmune diseases deserve a more intensive risk
evaluation. People whose occupations include public safety (eg
pilots or truck or bus drivers, workers) or professional athletes
are usually periodically tested to assess the ECG with a TFO test
and assess possible heart disease, including CAD, although there is
insufficient data to justify this. However, these evaluations can be
performed for medical-legal reasons. The threshold for performing a
visualization stress test for CAD in such persons is lower than in
the average patient.
REFRACTORY ANGINA PEKTORIS (RAP)
RAP as a form of chronic coronary syndrome (CCS) is defined as a
chronic condition caused by clinically established reversible
ischemia in the presence of CAD, which cannot be adequately
controlled by a combination of medical therapy, angioplasty with
stenting (PCI) or surgical coronary revascularization (CABG) [1 ]:
The following treatment options are considered:
1- Forced external counterpulsation (EECP) should be considered to
alleviate and minimize discomfort in patients with AP Refractory to
optimal pharmacological therapy and revascularization strategy
2-Transcutaneous electrical nerve stimulation (TENS) can be used to
alleviate the symptoms of refractory AP on optimal pharmacotherapy
and revascularization strategy
3-Spinal cord stimulation (SCS) can be used to alleviate the
symptoms of refractory angina to optimal pharmacol Th and
revascularization strategy
4-Transmyocardial revascularization (TMR) is not indicated in
patients with symptoms of refractory angina on optimal
pharmacological Th and revascularization strategy
FUTURE PERSPECTIVES OF CHRONIC CORONARY SYNDROMES CCS - Quote from
Brownwald's textbook of cardiology, authors David Morrow, J. J. A.
De Lemos and William Boden [135]:
Our understanding of CHRONIC CORONARY SYNDROMES (CCS), both as a
cause, optimal approach and treatment, is constantly evolving.
1. Complex and probably heterogeneous causes of myocardial ischemia
require continuous multidisciplinary research through experimental
studies in genetics, molecular biology, biochemistry, morphological
and functional aspects of coronary circulation, and pathological
morphology and pathophysiology of myocardial ischemia, which should
then be confirmed in clinical studies with sufficient statistical
power to generate new guidelines for more precise and simpler
diagnosis and effective therapy of CCS. Today, we are confronted
with the essential data that challenge the paradigm that Ischemic
CAD requires critical coronary atherosclerosis of the subepicardial
coronary artery or other structural heart disease that results in a
dramatic increase in oxygen consumption. Preclinical, translational
and clinical epidemiological data demonstrate abnormalities in
coronary artery function, which can lead to myocardial ischemia in
the absence of atherosclerotic obstruction.
2. However, so far, the treatments proposed for this important
syndrome-CHRONIC CORONARY SYNDROME have proved insufficient.
Additional insight into the pathobiology of ischemia could lead to
new directions in treatment.
3. An initial approach to targeted secondary-preventive therapy and
coronary revascularization, when necessary, is the best approach for
most patients with CCS. There are subgroups of patients with
high-risk indicators for whom coronary myocardial revascularization
should be logical. However, clinical controversy and doubt remains
as to whether such patients, including those with moderate or severe
ischemia on a noninvasive test, should be routinely subjected to
coronary myocardial revascularization in the absence of symptoms of
refractory to optimal preventive and pharmacological therapy (OMT).
4.. Definitive evidence for the care of patients with stable CCS and
other consequences of ischemia, especially left ventricular
dysfunction and mitral ischemic regurgitation, remains incomplete.
In our opinion, complete myocardial revascularization, usually
surgical -CABG, remains a reasonable option for patients with
multivessel CAD, LC dysfunction, and viable myocardium, especially
when there is objective evidence of ischemia. However, some recent
studies call this view into question. Despite our good experience
with CCS, there are no answers to important questions [135].
FUTURE PERSPECTIVES OF CHRONIC CORONARY SYNDROME CCS - quote from
Braunald's textbook of cardiology, authors David Morrow and William
Boden [181]: Our understanding of CCS, as a cause, optimal approach
and treatment, is constantly evolving.
1. Complex and probably heterogeneous causes of myocardial ischemia
require continuous multidisciplinary research through experimental
studies in genetics, molecular biology, biochemistry, morphological
and functional aspects of coronary circulation, and pathological
morphology and pathophysiology of myocardial ischemia, which should
then be confirmed randomly studies with sufficient statistical power
to generate new guidelines for more precise and simpler diagnosis
and effective therapy of CCS. Today, we are confronted with the
essential data that challenge the paradigm that Ischemic CAD
requires critical coronary atherosclerosis of the subepicardial
coronary artery or other structural heart disease that results in a
dramatic increase in oxygen consumption. Preclinical, translational
and clinical epidemiological data demonstrate abnormalities in
coronary artery function, which can lead to myocardial ischemia in
the absence of atherosclerotic obstruction.
2. However, so far, the treatments proposed for this important
syndrome-CHRONIC CORONARY SYNDROME have proved insufficient.
Additional insight into the pathobiology of ischemia could lead to
new directions in Th.
3. An initial approach to guided-targeted secondary-preventive
therapy and coronary revascularization, when necessary, is the best
approach for most patients with CCS. There are subgroups of patients
with high-risk indicators for whom coronary myocardial
revascularization should be logical. However, clinical controversy
and doubt remains as to whether such patients, including those with
moderate or severe ischemia on a noninvasive test, should routinely
undergo coronary myocardial revascularization in the absence of
symptoms of refractory to optimal preventive and pharmacological
therapy (OMT).
4.. Definitive evidence for the care of patients with stable CCS and
other consequences of ischemia, especially left ventricular
dysfunction and mitral ischemic regurgitation, remains incomplete.
In our opinion, complete myocardial revascularization, usually
surgical -CABG, remains a reasonable option for pts with multivessel
CAD, LC dysfunction, and viable myocardium, especially when there is
objective evidence of ischemia. However, some recent studies call
this view into question. Despite our good experience with CCS, there
are no answers to important questions [181].
CONCLUSION:
Careful and studious evaluation of the anamnesis, including
characterization of anginal symptoms and assessment of risk factors
and manifestations of cardiovascular diseases, as well as
appropriate physical examination and basic supplementary, basic
examination is essential for the diagnosis of chronic coronary
syndromes. If obstructive coronary artery disease (mean pre-test
probability) cannot be ruled out by clinical evaluation alone,
noninvasive diagnostic functional imaging tests with physical or
pharmacological loading or anatomical imaging should be used as the
initial test to exclude or diagnose chronic coronary syndromes
(clinical scenario 1). via coronary CT angiography. The selection of
the initial non-invasive diagnostic test is based on the probability
test for obstructive coronary heart disease (PTP), its clinical
probability, characteristics and test availability. An anatomical
and functional evaluation should be performed to decide on
revascularization. Either non-invasive or invasive functional
evaluation is required to assess the size of myocardial ischemia
associated with angiographic coronary artery stenosis, except for
very high-grade stenosis (> 90% of diameter stenosis). The risk
assessment for adverse events and mortality serves to determine for
which patients with CCS the prognostic benefit of revascularization
is predicted.
Assessment of left ventricular function by echocardiography is a
mandatory part of risk stratification. Patients at high risk of
adverse events undergo invasive examination to consider
revascularization, even when asymptomatic. The application of a
healthy lifestyle reduces the risk of subsequent CV events and
mortality and is always included in the program of appropriate
measures and therapy of secondary prevention. Clinicians should
advise and encourage the necessary lifestyle changes at each
clinical encounter with patients. Cognitive-behavioral psychological
interventions such as supporting patients to set realistic goals,
self-control, planning to implement change, coping with difficult
situations, adapting to the environment, and including social
support are effective behavioral change interventions.
Multidisciplinary teams can support patients to change to a healthy
lifestyle and guide them to avoid risk and risky behavior.
Anti-ischemic treatment must be tailored to the individual patient
based on comorbidity, concomitant therapy, expected tolerance and
adherence to therapy, but also the patient's preference. The choice
of anti-ischemic drugs for the treatment of CCS should be adjusted
to heart rate, blood pressure and left ventricular function.
Beta-blockers and / or calcium antagonists remain first-line drugs
in patients with CCS. Antithrombotic therapy is a key part of
secondary prevention in patients with CCS and requires careful
consideration. Long-term (and after 12 months) dual antiplatelet
therapy (DAPT) with aspirin or any P2I12 inhibitor should be
considered in patients with previous myocardial infarction, with or
without revascularization, who are at high risk of ischemic events
and low risk of severe or fatal bleeding. or rivaroxaban with very
low doses, unless they have an indication for oral anticoagulant
therapy (OAK) such as the presence of atrial fibrillation (AF).
Statins are recommended for all patients with CCS, regardless of the
level of LDL cholesterol. ACE inhibitors (or ARBs) are recommended
in the presence of heart failure (SI), diabetes, or hypertension and
should be considered in all high-risk patients. Proton pump
inhibitors are recommended in patients receiving aspirin or a
combination of antithrombotic therapies that are at high risk for
gastrointestinal bleeding. Efforts should be made to explain to
patients the importance of taking prescribed medication regularly,
based on evidence that regular adherence to treatment prevents
adverse events, relieves the patient of pain, and improves quality
of life. Repeated therapeutic education of patients is essential for
every clinical encounter. Patients with a long-term diagnosis of CCS
should visit a doctor periodically to assess possible changes in
risk status, adhere to treatment goals, and develop comorbidities.
Repeated physical or pharmacological stress tests, preferably
imaging stress tests, or invasive coronary angiography with
functional testing in case of worsening symptoms and / or increased
risk status are recommended. Assessment of the function and
dimensions of the heart cavities, myocardium and valves, as well as
a functional test to rule out significant asymptomatic (silent)
myocardial ischemia, should be considered every 3–5 years in
asymptomatic patients with a long-term diagnosis of CCS. Assessment
of coronary vasomotor function should be considered in patients
without obstructive coronary disease or with minor epicardial
coronary disease who have objective evidence of myocardial ischemia.
Objectives of treatment of chronic coronary symptoms (CCS):
improving prognosis, ie. reducing mortality by reducing the risk of
progression of atherosclerosis and preventing acute coronary events
and sudden death; and minimizing symptoms while improving quality of
life. Efforts should be made to explain to patients the importance
of evidence-based guidelines and guidelines for adherence to
treatment.
All patients with stable coronary heart disease need lifestyle
changes, risk factor reduction and pharmacological therapy, but all
patients with coronary artery stenosis do not benefit from
revascularization, which depends on the size of the ischemia and the
anatomical involvement of the coronary arteries. Optimal medical
therapy is the definitive therapy for patients with stable coronary
heart disease and low risk of cardiovascular events.
Revascularization and optimal medical therapy should be considered
as complementary rather than competitive treatments.
In high-risk CCS patients with mortality> 3% per year or AP
refractory to OMT, evidence of physical or pharmacological stress
ischemia is required, preferably stress echocardiography to
determine the size of ischemia on the stress echo test and
anatomical assessment to determine coronary artery disease. to
indicate revascularization leading to clinical benefit.
|
|
|
|
| |
|
|
REFERENCES:
- Juhani Knuuti (Chairperson), William Wijns (Chairperson),
Antti Saraste, Davide Capodanno, Emanuele Barbato, Christian
Funck-Brentano, Eva Prescott, Robert F. Storey, Christi Deaton,
Thomas Cuisset et al. 2019 ESC Guidelines for the diagnosis and
management of chronic coronary syndromes. European Heart Journal
2019:00, 1-71. ESC guidelines doi:10.1093/eurheartj/ehz425
- Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci
C, Bueno H, Caforio ALP, Crea F, et al. 2017 ESC Guidelines for
the management of acute myocardial infarction in patients
presenting with ST-segment elevation: The Task Force for the
management of ESC Guidelines. the European Society of Cardiology
(ESC). Eur Heart J 2018; 39:119-177.
- Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M,
Andreotti F, Bax JJ, et al. 2015 ESC Guidelines for the
management of acute coronary syndromes in patients presenting
without persistent ST-segment elevation: Task Force for the
Management of Acute Coronary Syndromes in Patients Presenting
without Persistent ST-Segment Elevation of the European Society
of Cardiology (ESC). Eur Heart J 2016; 37:267-315.
- Gilles Montalescot, Udo Sechtem, Stephan Achenbach, Felicita
Andreotti, Chris Arden, Andrzej Budaj et al. 2013 ESC guidelines
on the management of stable coronary artery disease. European
Heart Journal 2013; 34:2949-3003.
- Bastać D, Milošević A, Radulović N, et al. Incidenca i
karakteristike akutnog infarka miokarda u Zdravstvenom centru
Zaječar u periodu 1980-2000. ČASOPIS URGENTNE MEDICINE 2002;
Suppl 1:4-5.
- Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano
AL et al. 2016 European Guidelines on cardiovascular disease
prevention in clinical practice: The Sixth Joint Task Force of
the European Society of Cardiology and Other Societies on
Cardiovascular Disease Prevention in Clinical Practice Developed
with the special contribution of the European Association for
Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J
2016; 37:2315-2381.
- Ong P, Camici PG, Beltrame JF, Crea F, Shimokawa H, Sechtem
U, Kaski JC, Bairey Merz CN; Coronary Vasomotion Disorders
International Study Group (COVADIS). International
standardization of diagnostic criteria for microvascular angina.
Int J Cardiol 2018; 250:1620.
- Mygind ND, Michelsen MM, Pena A, Frestad D, Dose N, Aziz A,
et al. Coronary microvascular function and cardiovascular risk
factors in women with angina pectoris and no obstructive
coronary artery disease: the iPOWER study. J Am Heart Assoc
2016; 5:e003-064.
- Crea F, Camici PG, Bairey Merz CN. Coronary microvascular
dysfunction: an update. Eur Heart J 2014; 35:1101-1111.
- Beltrame JF, Crea F, Kaski JC, Ogawa H, Ong P, Sechtem U,
Shimokawa H, Bairey Merz CN. Coronary Vasomotion Disorders
International Study Group (COVADIS). International
standardization of diagnostic criteria for vasospastic angina.
Eur Heart J 2017; 38:2565-2568.
- Ong P, Athanasiadis A, Perne A, Mahrholdt H, Schaufele T,
Hill S, Sechtem U. Coronary vasomotor abnormalities in patients
with stable angina after successful stent implantation but
without in-stent restenosis. Clin Res Cardiol 2014; 103:11-19.
- Tsuburaya R, Takahashi J, Nakamura A, Nozaki E, Sugi M,
Yamamoto Y, et al. NOVEL Investigators. Beneficial effects of
longacting nifedipine on coronary vasomotion abnormalities after
drug-eluting stent implantation: the NOVEL study. Eur Heart J
2016; 37:2713-2721.
- JCS Joint Working Group. Guidelines for diagnosis and
treatment of patients with vasospastic angina (Coronary Spastic
Angina) (JCS 2013). Circ J 2014; 78:2779-2801.
- A De Vita A, Milo M, Sestito A, Lamendola P, Lanza GA, Crea
F. Association of coronary microvascular dysfunction with
restenosis of left anterior descending coronary artery disease
treated by percutaneous intervention. Int J Cardiol. 2016 Jun
14; 219:322-325.
- Reeh J, Therming CB, Heitmann M, Hojberg S, Sorum C, Bech J,
Husum D, Dominguez H, Sehestedt T, Hermann T, Hansen KW,
Simonsen L, Galatius S, Prescott E. Prediction of obstructive
coronary artery disease and prognosis in patients with suspected
stable angina. Eur Heart J 2018; 40:1426-1435.
- Maron DJ, Boden WE, O’Rourke RA, Hartigan PM, Calfas KJ,
Mancini GB, et al. COURAGE Trial Research Group. Intensive
multifactorial intervention for stable coronary artery disease:
optimal medical therapy in the COURAGE (Clinical Outcomes
Utilizing Revascularization and Aggressive Drug Evaluation)
trial. J Am Coll Cardiol 2010; 55:1348-1358.
- Rotenstein LS, Huckman RS, Wagle NW. Making patients and
doctors happier -the potential of patient-reported outcomes. N
Engl J Med 2017; 377:1309-1312.
- Chow CK, Jolly S, Rao-Melacini P, Fox KA, Anand SS, Yusuf S.
Association of diet, exercise, and smoking modification with
risk of early cardiovascular events after acute coronary
syndromes. Circulation 2010; 121:750-758.
- Booth JN III, Levitan EB, Brown TM, Farkouh ME, Safford MM,
Muntner P. Effect of sustaining lifestyle modifications
(nonsmoking, weight reduction, physical activity, and
mediterranean diet) after healing of myocardial infarction,
percutaneous intervention, or coronary bypass (from the REasons
for Geographic and Racial Differences in Stroke Study). Am J
Cardiol 2014; 113:1933-1940.
- Giannuzzi P, Temporelli PL, Marchioli R, Maggioni AP,
Balestroni G, Ceci V et al. GOSPEL Investigators. Global
secondary prevention strategies to limit event recurrence after
myocardial infarction: results of the GOSPEL study, a
multicenter, randomized controlled trial from the Italian
Cardiac Rehabilitation Network. Arch Intern Med 2008;
168:2194-2204.
- Keteyian SJ, Brawner CA, Savage PD, Ehrman JK, Schairer J,
Divine G, Aldred H, Ophaug K, Ades PA. Peak aerobic capacity
predicts prognosis in patients with coronary heart disease. Am
Heart J 2008; 156:292-300.
- Bastać D. Novi aspekti dijagnostičke i prognostičke procene
stabilne koronarne bolesti –revidirani dijagnostički i
prognostički algoritmi u 3 koraka. Zbornik abstrakta
simpozijuma: STABILNA KORONARNA BOLEST- Šta novo donosi evropski
vodič 2013, Zaječar, 2014.god, strana 6-16.
- Williams RP, Manou-Stathopoulou V, Redwood SR, Marber MS.
‘Warm-up Angina’: harnessing the benefits of exercise and
myocardial ischaemia. Heart 2014; 100:106-114.
- Cohn PF, Fox KM, Daly C. Silent myocardial ischemia.
Circulation 2003; 108:1263-1277.
- Davies RF, Goldberg AD, Forman S, Pepine CJ, Knatterud GL,
Geller N, et al. Asymptomatic Cardiac Ischemia Pilot (ACIP)
study two-year follow-up: outcomes of patients randomized to
initial strategies of medical therapy versus revascularization.
Circulation 1997; 95:2037-2043.
- Stone PH, Chaitman BR, Forman S, Andrews TC, Bittner V,
Bourassa MG. Prognostic significance of myocardial ischemia
detected by ambulatory electrocardiography, exercise treadmill
testing, and electrocardiogram at rest to predict cardiac events
by one year (the Asymptomatic Cardiac Ischemia Pilot
[ACIP]study). Am J Cardiol 1997; 80:1395-1401.
- Dušan Bastać. Uticaj veličine QRS skora na težinu
ventrikularnih aritmija u akutnoj fazi infarkta miokarda.
Timocki Med. Glasnik 1989; 14:229-233.
- Androulakis A, Aznaouridis KA, Aggeli CJ, Roussakis GN,
Michaelides AP, Kartalis AN. Transient ST-segment depression
during paroxysms of atrial fibrillation in otherwise normal
individuals: relation with underlying coronary artery disease. J
Am Coll Cardiol 2007; 50:1909-1911.
- Pradhan R, Chaudhary A, Donato AA. Predictive accuracy of ST
depression during rapid atrial fibrillation on the presence of
obstructive coronary artery disease. Am J Emerg Med 2012;
30:1042-1047.
- Raščanin Anastasija, Aranđelović Ivana, Bastać Mila, Bastać
Dušan. Uticaj metaboličkog sindroma na strukturne anomalije,
sistolnu i dijastolnu funkciju leve komore određivanu
ehokardiografijom u bolesnika sa atrijalnom fibrilacijom.
Timočki medicinski glasnik 2017; 42(3):132-138.
- Greenwood JP, Ripley DP, Berry C, McCann GP, Plein S,
Bucciarelli-Ducci et al. CE-MARC 2 Investigators. Effect of care
guided by cardiovascular magnetic resonance, myocardial
perfusion scintigraphy, or NICE guidelines on subsequent
unnecessary angiography rates: the CE-MARC 2 randomized clinical
trial. JAMA 2016; 316:1051-1060.
- Diamond GA, Forrester JS. Analysis of probability as an aid
in the clinical diagnosis of coronary-artery disease. N Engl J
Med 1979; 300:1350-1358.
- Foldyna B, Udelson JE, Karady J, Banerji D, Lu MT, Mayrhofer
T, et al. Pretest probability for patients with suspected
obstructive coronary artery disease: re-evaluating
Diamond-Forrester for the contemporary era and clinical
implications: insights from the PROMISE trial. Eur Heart J
Cardiovasc Imaging 2018; 20:574 581.
- Genders TS, Steyerberg EW, Alkadhi H, Leschka S, Desbiolles
L, Nieman K et al. A clinical prediction rule for the diagnosis
of coronary artery disease: validation, updating, and extension.
Eur Heart J 2011; 32:1316-1330.
- Daly C, Norrie J, Murdoch DL, Ford I, Dargie HJ, Fox K;
TIBET (Total Ischaemic Burden European Trial) study group. The
value of routine non-invasive tests to predict clinical outcome
in stable angina. Eur Heart J 2003; 24:532-540.
- Budoff MJ, Mayrhofer T, Ferencik M, Bittner D, Lee KL, Lu
MT, Coles A, Jang J, Krishnam M, Douglas PS, Hoffmann U. PROMISE
Investigators. Prognostic value of coronary artery calcium in
the PROMISE study (Prospective Multicenter Imaging Study for
Evaluation of Chest Pain). Circulation 2017; 136:1993-2005.
- Villines TC, Hulten EA, Shaw LJ, Goyal M, Dunning A,
Achenbach S, et al. CONFIRM Registry Investigators. Prevalence
and severity of coronary artery disease and adverse events among
symptomatic patients with coronary artery calcification scores
of zero undergoing coronary computed tomography angiography:
results from the CONFIRM (Coronary CT Angiography Evaluation for
Clinical Outcomes: An International Multicenter registry. J Am
Coll Cardiol. 2011; 58:2533-2540.
- Juarez-Orozco LE, Saraste A, Capodanno D, Prescott E, Ballo
H, Bax JJ, Wijns W, Knuuti J. Impact of a decreasing pre-test
probability on the performance of diagnostic tests for coronary
artery disease. Eur Heart J Cardiovasc Imaging 2019; doi:
10.1093/ehjci/jez054.
- Versteylen MO, Joosen IA, Shaw LJ, Narula J, Hofstra L.
Comparison of Framingham, PROCAM, SCORE, and Diamond Forrester
to predict coronary atherosclerosis and cardiovascular events. J
Nucl Cardiol 2011; 18:904-911.
- Fordyce CB, Douglas PS, Roberts RS, Hoffmann U, Al-Khalidi
HR, Patel MR, Granger CB, Kostis J, Mark DB, Lee KL, Udelson JE;
Prospective Multicenter Imaging Study for Evaluation of Chest
Pain (PROMISE) Investigators. Identification of patients with
stable chest pain deriving minimal value from noninvasive
testing: the PROMISE minimal-risk tool, a secondary analysis of
a randomized clinical trial. JAMA Cardiol 2017; 2:400 408.
- Jensen JM, Voss M, Hansen VB, Andersen LK, Johansen PB,
Munkholm H, Norgaard BL. Risk stratification of patients
suspected of coronary artery disease: comparison of five
different models. Atherosclerosis 2012; 220:557-562.
- Siontis GC, Mavridis D, Greenwood JP, Coles B,
Nikolakopoulou A, Juni P, Salanti G, Windecker S. Outcomes of
non-invasive diagnostic modalities for the detection of coronary
artery disease: network meta-analysis of diagnostic randomized
controlled trials. BMJ 2018; 360: k504.
- De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA,
Piroth Z, Jagic N, Mobius-Winkler S, Rioufol G, Witt N, Kala P,
MacCarthy P, Engstrom T, Oldroyd KG, Mavromatis K, Manoharan G,
Verlee P, Frobert O, Curzen N, Johnson JB, Juni P, Fearon WF.
FAME 2 Trial Investigators. Fractional flow reserve-guided PCI
versus medical therapy in stable coronary disease. N Engl J Med.
2012; 367:991-1001.
- Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’ t
Veer M, Klauss V, Manoharan G, Engstrom T, Oldroyd KG, Ver Lee
PN, MacCarthy PA, Fearon WF; FAME Study Investigators.
Fractional flow reserve versus angiography for guiding
percutaneous coronary intervention. N Engl J Med 2009;
360:213-224.
- Knuuti J, Ballo H, Juarez-Orozco LE, Saraste A, Kolh P,
Rutjes AWS, Juni P. Windecker S, Bax JJ, Wijns W. The
performance of non-invasive tests to rule-in and rule-out
significant coronary artery stenosis in patients with stable
angina: a meta-analysis focused on post-test disease
probability. Eur Heart J 2018; 39:3322-3330.
- Tonino PA, Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA,
Ver Lee PN, Maccarthy PA, Van’t Veer M, Pijls NH. Angiographic
versus functional severity of coronary artery stenoses in the
FAME study fractional flow reserve versus angiography in
multivessel evaluation. J Am Coll Cardiol 2010; 55:2816-2821.
- Hoffmann U, Ferencik M, Udelson JE, Picard MH, Truong QA,
Patel MR, Huang M, Pencina M, Mark DB, Heitner JF, Fordyce CB,
Pellikka PA, Tardif JC, Budoff M, Nahhas G, Chow B, Kosinski AS,
Lee KL, Douglas PS; PROMISE Investigators. Prognostic value of
noninvasive cardiovascular testing in patients with stable chest
pain: insights from the PROMISE trial (Prospective Multicenter
Imaging Study for Evaluation of Chest Pain). Circulation
2017;135: 2320-2332.
- Dusan Bastac, Zoran Joksimović et al. Dipirydamole stress
echocardiography in the evaluation of myocardial ischemia in
obese subjects with hypertension. European Journal of
Echocardiography 2002; 3 (Suppl I).
- Mitov V, Aleksic Z, Paunkovic N, Bastać D. Myocardial
perfusion scintigraphy in selection of patients with positive
and inconclusive finding of ECG exercise stress tests. European
Heart Journal 2007; 28(Supp l):625.
- Lubbers M, Dedic A, Coenen A, Galema T, Akkerhuis J, Bruning
T, Krenning B, Musters P, Ouhlous M, Liem A, Niezen A, Hunink M,
de Feijter P, Nieman K. Calcium imaging and selective computed
tomography angiography in comparison to functional testing for
suspected coronary artery disease: the multicentre randomized
CRESCENT trial. Eur Heart J 2016; 37:1232-1243.
- Gueret P, Deux JF, Bonello L, Sarran A, Tron C, Christiaens
L. Diagnostic performance of computed tomography coronary
angiography (from the prospective national multicenter
multivendor EVASCAN study). Am J Cardiol 2013; 111:471-478.
- Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP,
Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Juni P,
Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ,
Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R,
Zembala MO. 2018 ESC/EACTS Guidelines on myocardial
revascularization. Eur Heart J 2019; 40:87-165.
- Escaned J, Echavarria-Pinto M, Garcia-Garcia HM, van de Hoef
TP, de Vries T, Kaul P, ADVISE II Study Group. Prospective
assessment of the diagnostic accuracy of instantaneous wave-free
ratio to assess coronary stenosis relevance: results of ADVISEII
international, multicenter study (ADenosine Vasodilator
Independent Stenosis Evaluation II). JACC Cardiovasc Interv
2015; 8:824-833.
- Toth G, Hamilos M, Pyxaras S, Mangiacapra F, Nelis O, De
Vroey F, et al. Evolving concepts of angiogram: fractional flow
reserve discordances in 4000 coronary stenoses. Eur Heart J
2014; 35:2831-2838.
- Jeremias A, Maehara A, Genereux P, Asrress KN, Berry C, De
Bruyne B, et al. Multicenter core laboratory comparison of the
instantaneous wave-free ratio and resting Pd/Pa with fractional
flow reserve: the RESOLVE study. J Am Coll Cardiol 2014;
63:1253-1261.
- Van Belle E, Rioufol G, Pouillot C, Cuisset T, Bougrini K,
Teiger E, et al; Investigators of the Registre Franc¸ais de la
FFRR3F. Outcome impact of coronary revascularization strategy
reclassification with fractional flow reserve at time of
diagnostic angiography: insights from a large French multicenter
fractional flow reserve registry. Circulation 2014; 129:173-185.
- Toth G, Hamilos M, Pyxaras S, Mangiacapra F, Nelis O, De
Vroey F, et al. Evolving concepts of angiogram: fractional flow
reserve discordances in 4000 coronary stenoses. Eur Heart J
2014; 35:2831-2838.
- Julien Adjedj, Bernard De Bruyne, Vincent Floré, Giuseppe Di
Gioia, Angela Ferrara, Mariano Pellicano et al. Significance of
Intermediate Values of Fractional Flow Reserve in Patients with
Coronary Artery Disease. Circulation 2016;133(5):502-8.
- Collet C, Onuma Y, Andreini D, Sonck J, Pompilio G, Mushtaq
S, et al. Coronary computed tomography angiography for heart
team decision-making in multivessel coronary artery disease. Eur
Heart J 2018; 39:3689-3698.
- Maron DJ, Boden WE, O’Rourke RA, Hartigan PM, Calfas KJ,
Mancini GB, et al. COURAGE Trial Research Group. Intensive
multifactorial intervention for stable coronary artery disease:
optimal medical therapy in the COURAGE (Clinical Outcomes
Utilizing Revascularization and Aggressive Drug Evaluation)
trial. J Am Coll Cardiol 2010; 55:1348-1358.
- Chow CK, Jolly S, Rao-Melacini P, Fox KA, Anand SS, Yusuf S.
Association of diet, exercise, and smoking modification with
risk of early cardiovascular events after acute coronary
syndromes. Circulation 2010; 21:750-758.
- Keteyian SJ, Brawner CA, Savage PD, Ehrman JK, Schairer J,
Divine G, Aldred H, Ophaug K, Ades PA. Peak aerobic capacity
predicts prognosis in patients with coronary heart disease. Am
Heart J 2008; 156:292-300.
- Prochaska JJ, Benowitz NL. The past, present, and future of
nicotine addiction therapy. Annu Rev Med 2016; 67:467-486.
- Barth J, Jacob T, Daha I, Critchley JA. Psychosocial
interventions for smoking cessation in patients with coronary
heart disease. Cochrane Database Syst Rev 2015; 7:CD006886.
- Freeman AM, Morris PB, Barnard N, Esselstyn CB, Ros E,
Agatston A, et al. Trending cardiovascular nutrition
controversies. J Am Coll Cardiol 2017; 69:1172-1187.
- Khan SS, Ning H, Wilkins JT, Allen N, Carnethon M, Berry JD,
Sweis RN, Lloyd-Jones DM. Association of body mass index with
lifetime risk of cardiovascular disease and compression of
morbidity. JAMA Cardiol 2018; 3:280-287.
- Pack QR, Rodriguez-Escudero JP, Thomas RJ, Ades PA, West CP,
Somers VK, Lopez-Jimenez F. The prognostic importance of weight
loss in coronary artery disease: a systematic review and
meta-analysis. Mayo Clin Proc 2014; 89:1368-1377.
- Fiuza-Luces C, Garatachea N, Berger NA, Lucia A. Exercise is
the real polypill. Physiology (Bethesda) 2013; 28:330-358.
- Bruning RS, Sturek M. Benefits of exercise training on
coronary blood flow in coronary artery disease patients. Prog
Cardiovasc Dis 2015; 57:443-453.
- Cheng W, Zhang Z, Cheng W, Yang C, Diao L, Liu W.
Associations of leisuretime physical activity with
cardiovascular mortality: a systematic review and meta-analysis
of 44 prospective cohort studies. Eur J Prev Cardiol 2018;
25:1864-1872.
- Keteyian SJ, Brawner CA, Savage PD, Ehrman JK, Schairer J,
Divine G, Aldred H, Ophaug K, Ades PA. Peak aerobic capacity
predicts prognosis in patients with coronary heart disease. Am
Heart J 2008; 156:292-300.
- Lahtinen M, Toukola T, Junttila MJ, Piira OP, Lepojarvi S,
Kaariainen M, Huikuri HV, Tulppo MP, Kiviniemi AM. Effect of
changes in physical activity on risk for cardiac death in
patients with coronary artery disease. Am J Cardiol 2018;
121:143-148.
- Stewart RAH, Held C, Hadziosmanovic N, Armstrong PW, Cannon
CP, Granger CB, et al; STABILITY Investigators. Physical
activity and mortality in patients with stable coronary heart
disease. J Am Coll Cardiol 2017; 70:1689-1700.
- Anderson L, Thompson DR, Oldridge N, Zwisler AD, Rees K,
Martin N, Taylor RS. Exercise-based cardiac rehabilitation for
coronary heart disease. Cochrane Database Syst Rev
2016;1:CD001800.
- Rauch B, Davos CH, Doherty P, Saure D, Metzendorf MI,
Salzwedel A, Voller H, Jensen K, Schmid JP. The prognostic
effect of cardiac rehabilitation in the era of acute
revascularization and statin therapy: A systematic review and
meta-analysis of randomized and non-randomized studies - The
Cardiac Rehabilitation Outcome Study (CROS). Eur J Prev Cardiol
2016; 23:1914-1939.
- de Vries H, Kemps HM, van Engen-Verheul MM, Kraaijenhagen
RA, Peek N. Cardiac rehabilitation and survival in a large
representative community cohort of Dutch patients. Eur Heart J
2015; 36:1519 -1528.
- Benzer W, Rauch B, Schmid JP, Zwisler AD, Dendale P, Davos
CH, Kouidi E, Simon A, Abreu A, Pogosova N, Gaita D, Miletic B,
Bonner G, Ouarrak T, McGee H; EuroCaReD study group.
Exercise-based cardiac rehabilitation in twelve European
countries results of the European cardiac rehabilitation
registry. Int J Cardiol 2017; 228:58-67.
- Rudisch B, Nemeroff CB. Epidemiology of comorbid coronary
artery disease and depression. Biol Psychiatry 2003; 54:227-240.
- Baumeister H, Hutter N, Bengel J. Psychological and
pharmacological interventions for depression in patients with
coronary artery disease. Cochrane Database Syst Rev 2011;
9:CD008012.
- Richards SH, Anderson L, Jenkinson CE, Whalley B, Rees K,
Davies P, Bennett P, Liu Z, West R, Thompson DR, Taylor RS.
Psychological interventions for coronary heart disease: Cochrane
systematic review and meta-analysis. Eur J Prev Cardiol 2018;
25:247-259.
- Rutledge T, Redwine LS, Linke SE, Mills PJ. A meta-analysis
of mental health treatments and cardiac rehabilitation for
improving clinical outcomes and depression among patients with
coronary heart disease. Psychosom Med 2013; 75:335-349.
- Brook RD, Newby DE, Rajagopalan S. Air pollution and
cardiometabolic disease:an update and call for clinical trials.
Am J Hypertens 2017; 31:110.
- Munzel T, Schmidt FP, Steven S, Herzog J, Daiber A, Sorensen
M. Environmental noise and the cardiovascular system. J Am Coll
Cardiol 2018; 71:688-697.
- Steinke EE, Jaarsma T, Barnason SA, Byrne M, Doherty S,
Dougherty CM, et al. Sexual counselling for individuals with
cardiovascular disease and their partners: a consensus document
from the American Heart Association and the ESC Council on
Cardiovascular Nursing and Allied Professions (CCNAP). Eur Heart
J 2013;34:3217-3235.
- Stein R, Sardinha A, Araujo CG. Sexual activity and heart
patients: a contemporary perspective. Can J Cardiol 2016;
32:410-420.
- Chowdhury R, Khan H, Heydon E, Shroufi A, Fahimi S, Moore C,
Stricker B, Mendis S, Hofman A, Mant J, Franco OH. Adherence to
cardiovascular therapy: a meta-analysis of prevalence and
clinical consequences. Eur Heart J 2013; 34:2940-2948.
- Gnjidic D, Bennett A, Le Couteur DG, Blyth FM, Cumming RG,
Waite L, Handelsman D, Naganathan V, Matthews S, Hilmer SN.
Ischemic heart disease, prescription of optimal medical therapy
and geriatric syndromes in communitydwelling older men: a
population-based study. Int J Cardiol 2015; 192:49-55.
- Mohammed S, Arabi A, El-Menyar A, Abdulkarim S, AlJundi A,
Alqahtani A, Arafa S, Al Suwaidi J. Impact of polypharmacy on
adherence to evidence-based medication in patients who underwent
percutaneous coronary intervention. Curr Vasc Pharmacol 2016;
14:388-393.
- Wimmer BC, Cross AJ, Jokanovic N, Wiese MD, George J,
Johnell K, Diug B, Bell JS. Clinical outcomes associated with
medication regimen complexity in older people: a systematic
review. J Am Geriatr Soc 2017; 65:747-753.
- Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R,
Keepanasseril A, Agoritsas T, Mistry N, Iorio A, Jack S,
Sivaramalingam B, Iserman E, Mustafa RA, Jedraszewski D, Cotoi
C, Haynes RB. Interventions for enhancing medication adherence.
Cochrane Database Syst Rev 2014;11:CD000011.
- Demonceau J, Ruppar T, Kristanto P, Hughes DA, Fargher E,
Kardas P, De Geest S, Dobbels F, Lewek P, Urquhart J, Vrijens B;
ABC project team. Identification and assessment of
adherence-enhancing interventions in studies assessing
medication adherence through electronically compiled drug dosing
histories: a systematic literature review and meta-analysis.
Drugs 2013; 73:545-562.
- Husted SE, Ohman EM. Pharmacological and emerging therapies
in the treatment of chronic angina. Lancet 2015; 386:691-701.
- National Institute for Health and Care Excellence (NICE).
Stable angina: management. Clinical guideline [CG126].
https://www.nice.org.uk/guidance/cg126 (28 March 2019).
- Klein WW, Jackson G, Tavazzi L. Efficacy of monotherapy
compared with combined antianginal drugs in the treatment of
chronic stable angina pectoris: a meta-analysis. Coron Artery
Dis 2002; 13:427-436.
- Rousan TA, Mathew ST, Thadani U. Drug therapy for stable
angina pectoris. Drugs 2017; 77:265-284.
- Pehrsson SK, Ringqvist I, Ekdahl S, Karlson BW, Ulvenstam G,
Persson S. Monotherapy with amlodipine or atenolol versus their
combination in stable angina pectoris. Clin Cardiol 2000;
23:763-770.
- Emanuelsson H, Egstrup K, Nikus K, Ellstrom J, Glud T, Pater
C, Scheibel M, Tisell A, Totterman KJ, Forsby M. Antianginal
efficacy of the combination of felodipine-metoprolol 10/100 mg
compared with each drug alone in patients with stable
effort-induced angina pectoris: a multicenter parallel group
study. The TRAFFIC Study Group. Am Heart J 1999; 137:854-862.
- Belsey J, Savelieva I, Mugelli A, Camm AJ. Relative efficacy
of antianginal drugs used as add-on therapy in patients with
stable angina: a systematic review and meta-analysis. Eur J Prev
Cardiol 2015; 22:837-848.
- Wight LJ, VandenBurg MJ, Potter CE, Freeth CJ. A large scale
comparative study in general practice with nitroglycerin spray
and tablet formulations in elderly patients with angina
pectoris. Eur J Clin Pharmacol 1992; 42:341-342.
- Diaz A, Bourassa MG, Guertin MC, Tardif JC. Long-term
prognostic value of resting heart rate in patients with
suspected or proven coronary artery disease. Eur Heart J 2005;
26:967-974.
- Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D,
Ducimetiere P. Heart-rate profile during exercise as a predictor
of sudden death. N Engl J Med 2005;3 52:1951-1958.
- Bangalore S, Bhatt DL, Steg PG, Weber MA, Boden WE, Hamm CW,
Montalescot G, Hsu A, Fox KA, Lincoff AM. beta-blockers and
cardiovascular events in patients with and without myocardial
infarction: post hoc analysis from the CHARISMA trial. Circ
Cardiovasc Qual Outcomes 2014; 7:872-881.
- Andersson C, Shilane D, Go AS, Chang TI, Kazi D, Solomon MD,
Boothroyd DB, Hlatky MA. b-blocker therapy and cardiac events
among patients with newly diagnosed coronary heart disease. J Am
Coll Cardiol 2014; 64:247-252.
- Hwang D, Lee JM, Kim HK, Choi KH, Rhee TM, Park J, Park TK,
Yang JH, Song YB, Choi JH, Hahn JY, Choi SH, Koo BK, Kim YJ,
Chae SC, Cho MC, Kim CJ, Gwon HC, Jeong MH, Kim HS; KAMIR
Investigators. Prognostic impact of betablocker dose after acute
myocardial infarction. Circ J 2019; 83:410-417.
- Dahl Aarvik M, Sandven I, Dondo TB, Gale CP, Ruddox V,
Munkhaugen J, Atar D, Otterstad JE. Effect of oral beta-blocker
treatment on mortality in contemporary post-myocardial
infarction patients: a systematic review and meta-analysis. Eur
Heart J Cardiovasc Pharmacother 2019; 5:1220.
- Bangalore S, Steg G, Deedwania P, Crowley K, Eagle KA, Goto
S, Ohman EM, Cannon CP, Smith SC, Zeymer U, Hoffman EB, Messerli
FH, Bhatt DL; REACH Registry Investigators. b-Blocker use and
clinical outcomes in stable outpatients with and without
coronary artery disease. JAMA 2012; 308:1340-1349.
- Padala SK, Lavelle MP, Sidhu MS, Cabral KP, Morrone D, Boden
WE, Toth PP. Antianginal therapy for stable ischemic heart
disease: a contemporary review. J Cardiovasc Pharmacol Ther
2017; 22:499-510.
- Poole-Wilson PA, Lubsen J, Kirwan BA, van Dalen FJ, Wagener
G, Danchin N, et al. Coronary disease Trial Investigating
Outcome with Nifedipine gastrointestinal therapeutic system
investigators. Effect of long-acting nifedipine on mortality and
cardiovascular morbidity in patients with stable angina
requiring treatment (ACTION trial): randomized controlled trial.
Lancet 2004; 364:849-857.
- Nissen SE, Tuzcu EM, Libby P, Thompson PD, Ghali M, Garza D,
Berman L, Shi H, Buebendorf E, Topol EJ; CAMELOT Investigators.
Effect of antihypertensive agents on cardiovascular events in
patients with coronary disease and normal blood pressure: the
CAMELOT study: a randomized controlled trial. JAMA 2004;
292:2217-2225.
- Heidenreich PA, McDonald KM, Hastie T, Fadel B, Hagan V, Lee
BK, Hlatky MA. Meta-analysis of trials comparing beta-blockers,
calcium antagonists, and nitrates for stable angina. JAMA 1999;
281:1927-1936.
- K Mohee, SB Wheatcroft. Optimal Medical Therapy and
Percutaneous Coronary Intervention for Stable Angina: Why
Patients Should 'Be Taking' and 'Keep Taking' the Tablets. J
Clin Pharm Ther. 2014; 39(4):331-3.
- Bastać D. Farmakološka terapija stabilne koronarne bolesti-u
fokusu podgrupe s terapijskim izazovom. Zbornik abstrakta
STABILNA KORONARNA BOLEST Šta novo donosi evropski vodič 2013,
Zaječar, 2014.god, strana 22-24.
- IONA Study Group. Effect of nicorandil on coronary events in
patients with stable angina: The Impact of Nicorandil in Angina
(IONA) randomised trial. Lancet 2002; 359:1269-1275.
- Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, Murphy SA,
Budaj A, Braunwald E et al. MERLIN-TIMI 36 Trial Investigators.
Effects of ranolazine on recurrent cardiovascular events in
patients with non-ST-elevation acute coronary syndromes: the
MERLIN-TIMI 36 randomized trial. JAMA 2007; 297:1775-1783.
- Wilson SR, Scirica BM, Braunwald E, Murphy SA,
Karwatowska-Prokopczuk E, et al. Efficacy of ranolazine in
patients with chronic angina observations from the randomized,
double-blind, placebo-controlled MERLIN-TIMI (Metabolic
Efficiency with Ranolazine for Less Ischemia in Non-ST-Segment
Elevation Acute Coronary Syndromes) 36 Trial. J Am Coll Cardiol
2009; 53:1510-1516.
- McCarthy CP, Mullins KV, Kerins DM. The role of
trimetazidine in cardiovascular disease: beyond an anti-anginal
agent. Eur Heart J Cardiovasc Pharmacother 2016; 2:266 -272.
- European Medicines Agency. Questions and answers on the
review of medicines containing trimetazidine (20 mg tablets, 35
mg modified release tablet and 20 mg/ml oral solution).
https://www.ema.europa.eu/en/documents/referral/
questions-answers-review-medicines-containing-trimetazidine-20-mg-tablets-
35-mg-modified-release/ml-oral-solution_en.pdf (28 March 2019).
- Noman A, Ang DS, Ogston S, Lang CC, Struthers AD. Effect of
high-dose allopurinol on exercise in patients with chronic
stable angina: a randomised, placebo-controlled crossover trial.
Lancet 2010; 375:2161-2167.
- Singh JA, Yu S. Allopurinol reduces the risk of myocardial
infarction (MI) in the elderly: a study of Medicare claims.
Arthritis Res Ther 2016; 18:209.
- Aviv A. Shaul and David Hasdai: Chronic ischaemic heart
disease: Pharmacological therapy. IN A. John Camm, Thomas F.
Lusher, Gerald Maurer and Patrick W. Serreys, editors. The ESC
Textbook of Cardiovascular Medicine, 3rd ed. Oxford Univesity
press; 2019. P.1387-1393.
- Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F,
Jeppsson A. 2017 ESC focused update on dual antiplatelet therapy
in coronary artery disease developed in collaboration with
EACTS: The Task Force for dual antiplatelet therapy in coronary
artery disease of the European Society of Cardiology (ESC) and
of the European Association for Cardio-Thoracic Surgery (EACTS).
Eur Heart J 2018; 39:213-260.
- Bhatt DL, Bonaca MP, Bansilal S, Angiolillo DJ, Cohen M,
Storey RF, Im K, Murphy SA, Held P, Braunwald E, Sabatine MS,
Steg PG. Reduction in ischemic events with ticagrelor in
diabetic patients with prior myocardial infarction in
PEGASUS-TIMI 54. J Am Coll Cardiol 2016; 67:2732-2740.
- Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode
C, et al. ATLAS ACS 2TIMI 51 Investigators. Rivaroxaban in
patients with a recent acute coronary syndrome. N Engl J Med
2012; 366:919.
- Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG,
Yusuf S et al. COMPASS Investigators. Rivaroxaban with or
without aspirin in stable cardiovascular disease. N Engl J Med
2017; 377:1319-1330.
- Al-Lamee R, Thompson D, Dehbi HM, Sen S, Tang K, Davies J,
et al; ORBITA investigators. Percutaneous coronary intervention
in stable angina (ORBITA): a doubleblind, randomised controlled
trial. Lancet 2018; 391:3140.
- Trikalinos TA, Alsheikh-Ali AA, Tatsioni A, Nallamothu BK,
Kent DM. Percutaneous coronary interventions for non-acute
coronary artery disease: a quantitative 20-year synopsis and a
network meta-analysis. Lancet 2009; 373:911-918.
- Stergiopoulos K, Brown DL. Initial coronary stent
implantation with medical therapy vs medical therapy alone for
stable coronary artery disease: meta-analysis of randomized
controlled trials. Arch Intern Med 2012; 172:312-319.
- Bangalore S, Pursnani S, Kumar S, Bagos PG. Percutaneous
coronary intervention versus optimal medical therapy for
prevention of spontaneous myocardial infarction in subjects with
stable ischemic heart disease. Circulation 2013; 127:769-781.
- Windecker S, Stortecky S, Stefanini GG, da Costa BR, Rutjes
AW, Di Nisio M, et al. Revascularisation versus medical
treatment in patients with stable coronary artery disease:
network meta-analysis. BMJ 2014; 348: g3859.
- Xaplanteris P, Fournier S, Pijls NHJ, Fearon WF, Barbato E,
Tonino PAL, et al. FAME 2 Investigators. Five-year outcomes with
PCI guided by fractional flow reserve. N Engl J Med 2018;
379:250-259.
- Franz-Josef Neumann, Miguel Sousa-Uva, Anders Ahlsson,
Fernando Alfonso, Adrian P. Banning (UK), Umberto Benedetto et
al. 2018 ESC/EACTS Guidelines on myocardial Revascularization.
The Task Force on myocardial revascularization of the
EuropeanSociety of Cardiology (ESC) and European Association for
Cardio-Thoracic Surgery (EACTS). European Heart Journal (2019)
40, 87–165. doi:10.1093/eurheartj/ehy394
- Mohee K, Wheatcroft S B. Optimal Medical Therapy and
Percutaneous Coronary Intervention for Stable Angina: Why
Patients Should 'Be Taking' and 'Keep Taking' the Tablets. J
Clin Pharm Ther. 2014;39(4):331-333.
- William E. Boden, M.D., Robert A. O'Rourke, M.D., Koon K.
Teo, M.B., B.Ch., Ph.D., Pamela M. Hartigan, Ph.D., David J.
Maron, M.D., William J. Kostuk, M.D., ET al. for the COURAGE
Trial Research Group. Optimal Medical Therapy with or without
PCI for Stable Coronary Disease N Engl J Med 2007;
356:1503-1516. DOI: 10.1056/NEJMoa070829.
- Bradley SM, Chan PS, Hartigan PM, Nallamothu BK, Weintraub
WS, Sedlis SP, Dada M, Maron DJ, Kostuk WJ, Berman DS, Teo KK,
Mancini GB, Boden WE, Spertus JA. Validation of the appropriate
use criteria for percutaneous coronary intervention in patients
with stable coronary artery disease (from the COURAGE trial). Am
J Cardiol. 2015 ;116(2):167-73. doi:
10.1016/j.amjcard.2015.03.057. Epub 2015 Apr 17.
- David Morow, James A. De Lemos, William E. Boden: Stable
ischemic heart disease: Future perspective. IN Douglas P. Zipes,
editor in chef. Braunwald’s Heart Disease.11th ed. Elsevier;
2019. p.1254.
- Mancini GBJ, Boden WE, Brooks MM, Vlachos H, Chaitman BR,
Frye R, Bittner V, Hartigan PM, Dagenais GR. Impact of treatment
strategies on outcomes in patients with stable coronary artery
disease and type 2 diabetes mellitus according to presenting
angina severity: A pooled analysis of three federally-funded
randomized trials. Atherosclerosis. 2018; 277:186-194. doi:
10.1016/j.atherosclerosis.2018.04.005. Epub 2018 Jun 1.
- David J. Maron, Judith S. Hochman, Harmony R. Reynolds,
Sripal Bangalore, Sean M. O’Brien, William E. Boden, M.D et al.,
for the ISCHEMIA Research GroupApril 9, 2020. Initial Invasive
or Conservative Strategy for Stable Coronary Disease. N Engl J
Med 2020; 382:1395-1407. DOI: 10.1056/NEJMoa191592
- International Study of Comparative Health Effectiveness with
Medical and Invasive Approaches - American College of Cardiology
4/21/2020.
https://www.acc.org/latest-in-cardiology/clinical-trials/2019/11/15/17/27/ischemia
1/6
- David J. Maron, Judith S. Hochman, Harmony R. Reynolds,
Sripal Bangalore. Initial Invasive or Conservative Strategy for
Stable Coronary Disease. N Engl J Med 2020; 382:1395-1407.
https://www.nejm.org/doi/full/10.1056/NEJMoa1915922 1/7
- Stephan D. Fihn et al. A Report of the American College of
Cardiology Foundation/American Heart Association Task Force on
Practice Guidelines, and the American College of Physicians,
American Association for Thoracic Surgery, Preventive
Cardiovascular Nurses Association, Society for Cardiovascular
Angiography and Interventions, and Society of Thoracic Surgeons
am vodič2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the
Diagnosis and Management of Patients With Stable Ischemic Heart
Disease. JACC 2012; 60:64-144.
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF,
Coats AJS et al. 2016 ESC Guidelines for the diagnosis and
treatment of acute and chronic heart failure: The Task Force for
the diagnosis and treatment of acute and chronic heart failure
of the European Society of Cardiology (ESC)Developed with the
special contribution of the Heart Failure Association (HFA) of
the ESC. Eur Heart J 2016; 37:2129-2200.
- Van Holten TC, Waanders LF, de Groot PG, Vissers J, Hoefer
IE, Pasterkamp G, Prins MW, Roest M. Circulating biomarkers for
predicting cardiovascular disease risk; a systematic review and
comprehensive overview of meta-analyses. PLoS One 2013; 8:
e62080.
- Hjalmarson A, Goldstein S, Fagerberg B, Wedel H, Waagstein
F, Kjekshus J, et al. Effects of controlled-releasemetoprolol on
total mortality, hospitalizations, and well-being in patients
with heart failure: the Metoprolol CR/XL Randomized Intervention
Trial in congestive heart failure (MERIT-HF). MERIT-HF Study
Group. JAMA 2000; 283:1295-1302.
- Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P,
et al. Carvedilol Prospective Randomized Cumulative Survival
Study Group. Effect of carvedilol on survival in severe chronic
heart failure. N Engl J Med 2001; 344:1651-1658.
- Dusan Bastać, Vladimir Mitov et al : Comparasion of the
efect of Carvediol versus Metoprolol on Sistolic and Diastolic
Left Ventricular Function in patients with Ischaemic Dilated
cardiomyopathy. Journal of American College of Cardiology 1998;
31:78 C (abstract).
- Packer M, Fowler MB, Roecker EB, Coats AJ, Katus HA, Krum H,
Mohacsi P, Rouleau JL, Tendera M, Staiger C, Holcslaw TL,
Amann-Zalan I, DeMets DL; Carvedilol Prospective Randomized
Cumulative Survival (COPERNICUS) Study Group. Effect of
carvedilol on the morbidity of patients with severe chronic
heart failure: results of the carvedilol prospective randomized
cumulative survival (COPERNICUS) study. Circulation 2002;
106:2194-2199.
- CIBIS-II Investigators and Committees. The Cardiac
Insufficiency Bisoprolol Study II (CIBIS-II): a randomised
trial. Lancet 1999; 353:913.
- Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ,
Parkhomenko A, Borbola J, Cohen-Solal A, Dumitrascu D, Ferrari
R, Lechat P, Soler-Soler J, Tavazzi L, Spinarova L, Toman J,
Bohm M, Anker SD, Thompson SG, Poole-Wilson PA; SENIORS
Investigators. Randomized trial to determine the effect of
nebivolol on mortality and cardiovascular hospital admission in
elderly patientsnwith heart failure (SENIORS). Eur Heart J 2005;
26:215-225.
- Bastać Dušan, Jelenković Bratimirka, Joksimović Zoran,
Aleksić Aleksandar. Šta je novo u dijagnostici i lečenju akutne
srčane insuficijencije? Timočki medicinski glasnik. 2015;40
(4):281-293.
- Packer M, O’Connor CM, Ghali JK, Pressler ML, Carson PE,
Belkin RN, et al. Effect of amlodipine on morbidity and
mortality in severe chronic heart failure. Prospective
Randomized Amlodipine Survival Evaluation Study Group. N Engl J
Med 1996; 335:1107-1114.
- Wijeysundera HC, Hansen MS, Stanton E, Cropp AS, Hall C,
Dhalla NS, Ghali J, Rouleau JL; PRAISE II Investigators.
Neurohormones and oxidative stress in nonischemic
cardiomyopathy: relationship to survival and the effect of
treatment with amlodipine. Am Heart J 2003; 146:291-297.
- Faris R, Flather M, Purcell H, Henein M, Poole-Wilson P,
Coats A. Current evidence supporting the role of diuretics in
heart failure: a meta analysis of randomized controlled trials.
Int J Cardiol 2002; 82:149-158.
- Dagenais GR, Pogue J, Fox K, Simoons ML, Yusuf S.
Angiotensin-convertingenzyme inhibitors in stable vascular
disease without left ventricular systolic dysfunction or heart
failure: a combined analysis of three trials. Lancet 2006;
368:581-588.
- Gage RM, Burns KV, Bank AJ. Echocardiographic and clinical
response to cardiac resynchronization therapy in heart failure
patients with and without previous right ventricular pacing. Eur
J Heart Fail 2014; 16:1199-1205.
- Lindholm D, Lindback J, Armstrong PW, Budaj A, Cannon CP.
Biomarker-based risk model to predict cardiovascular mortality
in patients with stable coronary disease. J Am Coll Cardiol
2017; 70:813-826.
- Eapen DJ, Manocha P, Patel RS, Hammadah M, Veledar E, Wassel
C, Nanjundappa RA, Sikora S, Malayter D, Wilson PW, Sperling L,
Quyyumi AA, Epstein SE. Aggregate risk score based on markers of
inflammation, cell stress, and coagulation is an independent
predictor of adverse cardiovascular outcomes. J Am Coll Cardiol
2013; 62:329-337.
- Bugiardini R, Bairey Merz CN. Angina with "normal" coronary
arteries: a changing philosophy. JAMA 2005; 293:477-484.
- Vermeltfoort IA, Raijmakers PG, Odekerken DA, Kuijper AF,
Zwijnenburg A, Teule GJ. Association between anxiety disorder
and the extent of ischemia observed in cardiac syndrome X. J
Nucl Cardiol 2009; 16:405-410.
- Ford TJ, Stanley B, Good R, Rocchiccioli P, McEntegart M,
Watkins S, et al. Stratified medical therapy using invasive
coronary function testing in angina: the CorMicA trial. J Am
Coll Cardiol 2018; 72:2841-2855.
- Bastac D. et al. Routine assesment of left ventricular
diastolic dysfunction in coronary artery disease by Doppler
exercise stress testing. European Journal of Echocardiography
2003; 4 (Suppl.1):96.
- Crea F, Camici PG, Bairey Merz CN. Coronary microvascular
dysfunction: an update. Eur Heart J 2014; 35:1101-1111.
- van de Hoef TP, van Lavieren MA, Damman P, Delewi R, Piek
MA, Chamuleau SA, Voskuil M, et al. Physiological basis and
long-term clinical outcome of discordance between fractional
flow reserve and coronary flow velocity reserve in coronary
stenoses of intermediate severity. Circ Cardiovasc Interv 2014;
7:301-311.
- Taqueti VR, Everett BM, Murthy VL, Gaber M, Foster CR,
Hainer J, Blankstein R, Dorbala S, Di Carli MF. Interaction of
impaired coronary flow reserve and cardiomyocyte injury on
adverse cardiovascular outcomes in patients without overt
coronary artery disease. Circulation 2015; 131:528-535.
- Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J,
Dorbala S, Blankstein R, Di Carli MF. Association between
coronary vascular dysfunction and cardiac mortality in patients
with and without diabetes mellitus. Circulation 2012;
126:1858-1868.
- Lee JM, Jung JH, Hwang D, Park J, Fan Y, Na SH, Doh JH, Nam
CW, Shin ES, Koo BK. Coronary flow reserve and microcirculatory
resistance in patients with intermediate coronary stenosis. J Am
Coll Cardiol 2016; 67:1158-1169.
- Lee JM, Choi KH, Hwang D, Park J, Jung JH, Kim HY, Jung HW,
Cho YK, Yoon HJ, Song YB, Hahn JY, Doh JH, Nam CW, Shin ES, Hur
SH, Koo BK. Prognostic implication of thermodilution coronary
flow reserve in patients undergoing fractional flow reserve
measurement. JACC Cardiovasc Interv 2018; 11:1423-1433.
- Radico F, Cicchitti V, Zimarino M, De Caterina R. Angina
pectoris and myocardial ischemia in the absence of obstructive
coronary artery disease: practical considerations for diagnostic
tests. JACC Cardiovasc Interv 2014; 7:453-463.
- Mejia-Renteria H, van der Hoeven N, van de Hoef TP,
Heemelaar J, Ryan N, Lerman A, van Royen N, Escaned J. Targeting
the dominant mechanism of coronary microvascular dysfunction
with intracoronary physiology tests. Int J Cardiovasc Imaging
2017; 33:1041-1059.
- Leung M, Juergens CP, Lo ST, Leung DY. Evaluation of
coronary microvascular function by left ventricular contractile
reserve with low-dose dobutamine echocardiography.
EuroIntervention 2014; 9:1202-1209.
- Sicari R, Rigo F, Cortigiani L, Gherardi S, Galderisi M,
Picano E. Additive prognostic value of coronary flow reserve in
patients with chest pain syndrome and normal or near-normal
coronary arteries. Am J Cardiol 2009; 103:626-631.
- Echavarria-Pinto M, Escaned J, Macias E, Medina M, Gonzalo
N, Petraco R, Sen S, Jimenez-Quevedo P, et al. Disturbed
coronary hemodynamics in vessels with intermediate stenoses
evaluated with fractional flow reserve: a combined analysis of
epicardial and microcirculatory involvement in ischemic heart
disease. Circulation 2013; 128:2557-2566.
- Nolte F, van de Hoef TP, Meuwissen M, Voskuil M, Chamuleau
SA, Henriques JP, Verberne HJ, van Eck-Smit BL, Koch KT, de
Winter RJ, Spaan JA, Tijssen JG, Siebes M, Piek JJ. Increased
hyperaemic coronary microvascular resistance adds to the
presence of myocardial ischaemia. EuroIntervention 2014;
9:1423-1431.
- Gutierrez E, Flammer AJ, Lerman LO, Elizaga J, Lerman A,
Fernandez-Aviles F. Endothelial dysfunction over the course of
coronary artery disease. Eur Heart J 2013; 34:3175-3181.
- Matsuzawa Y, Sugiyama S, Sugamura K, Nozaki T, Ohba K,
Konishi M, et al. Digital assessment of endothelial function and
ischemic heart disease in women. J Am Coll Cardiol 2010;
55:1688-1696.
- Beltrame JF, Crea F, Kaski JC, Ogawa H, Ong P, Sechtem U,
Shimokawa H, Bairey Merz CN. Coronary Vasomotion Disorders
International Study Group (COVADIS). International
standardization of diagnostic criteria for vasospastic angina.
Eur Heart J 2017; 38:2565-2568.
- Ong P, Athanasiadis A, Perne A, Mahrholdt H, Schaufele T,
Hill S, Sechtem U. Coronary vasomotor abnormalities in patients
with stable angina after successful stent implantation but
without in-stent restenosis. Clin Res Cardiol 2014; 103:1119.
- Tsuburaya R, Takahashi J, Nakamura A, Nozaki E, Sugi M,
Yamamoto Y, Hiramoto T, Horiguchi S, Inoue K, Goto T, Kato A,
Shinozaki T, Ishida E, Miyata S, Yasuda S, Shimokawa H. NOVEL
Investigators. Beneficial effects of longacting nifedipine on
coronary vasomotion abnormalities after drug-eluting stent
implantation: the NOVEL study. Eur Heart J 2016; 37:2713-2721.
- JCS Joint Working Group. Guidelines for diagnosis and
treatment of patients with vasospastic angina (Coronary Spastic
Angina) (JCS 2013). Circ J 2014; 78:2779-2801.
- Takagi Y, Yasuda S, Takahashi J, Tsunoda R, Ogata Y, Seki A,
Sumiyoshi T, Matsui M, Goto T, Tanabe Y, Sueda S, Sato T, Ogawa
S, Kubo N, Momomura S, Ogawa H, Shimokawa H; Japanese Coronary
Spasm Association. Clinical implications of provocation tests
for coronary artery spasm: safety, arrhythmic complications, and
prognostic impact: multicentre registry study of the Japanese
Coronary Spasm Association. Eur Heart J 2013;34 :258-267.
- David Morow, James A. De Lemos, William E. Boden. Stable
ischemic heart disease: Future perspective. In: Douglas P.
Zipes, editor in chef. Braunwald’s Heart Disease. 11th ed.
Elsevier; 2019. p. 1254.
|
|
|
|











