|
|
||||||||
| UDK 616.211085.238 | ISSN 03502899, 33(2008) br.12 p.22-27 | |||||||
| Original paper Mometasone furoate in the therapy of allergic rhinitisVesna Stojanović Kamberović(1), Goran Bjelogrlić(2), Snežana
Babac(3), Rade Kosanović(3) |
||||||||
|
|
||||||||
| Summary: INTRODUCTION: Acute rhinitis can be seasonal (intermittent) or perennial (persistent) and according to the ARIA (Allergic Rhinitis and its Impact on Asthma) Association it is the most significant risk factor in the onset of asthma. The incidence of seasonal allergic rhinitis is 10% of the overall population, and perennial 1020%. AIM: to show the efficiency of the mometasone furoate application in the therapy of allergic rhinitis. METHOD: The diagnosis of allergic rhinitis was based on anamnesis, clinical ENT examination, allergy tests and with the application of highly potent unfluorionated corticosteroid. RESULTS: During 2007 mometasone furoate, as nasal spray, was applied as monotherapy in 267 patients from 2 to 18 years of age, 89.51% of whom were schoolchildren. There were 164 male patients. There were 91 patients (31.98%) with seasonal rhinitis and 176 patients (68.92%) with perennial rhinitis. Grass and weed pollen were the dominant allergens in seasonal rhintis and dust mites in persistent rhinitis. The main symptoms were: sneezing, nose secretion, nasal obstruction, and nasal congestion. Because of the fast start of medication, after 57 hours, there was a significant decrease of symptoms and signs of ailment in a third of the patients, and after 72 hours the symptoms withdrew significantly in 167 patients (62.54%). After two weeks of medication application, 70% of the patients responded favourably. Sneezing, nasal congestion and itching in the nose were reduced in 61 patients (67.03%) with seasonal rhinitis, and nasal congestion, obstruction, and nasal secretion withdrew in 132 patients (75.56%) with perennial rhinitis. The medication was applied in a period of 2/4/6 months continually, or with pauses as per indications. CONCLUSION: Allergic rhinitis as a local manifestation of systematic allergic reaction was treated with mometasone furoate because of its superior systemic safety, high efficiency and extremely good local tolerance. The drug was applied as prophylaxis and therapy of seasonal rhinitis and therapy in persistent allergic rhinitis of varying etiology. Key words: allergic rhinitis, therapy, mometasone furoate Napomena: tekst rada na srpskom jeziku Note: text in Serbian |
||||||||
| INTRODUCTION Allergic rhinitis (AR) is the most common respiratory chronic disease, affecting 10-20% of the world population [1]. The number of affected individuals in the USA ranges between 20 and 40 million, out of which paediatric population accounts for approximately 40% while adult population accounts for 10-30% [2,3,6]. It may develop at any age, with alarmingly increasing incidence and prevalence among children. Generally, diagnosis of AR is established before the age of 20. The prevalence of AR-affected adults in Europe is approximately 23% [4]. The incidence rates of seasonal and perennial allergic rhinitis among the general population are 10% and 10-20%, respectively. Definition and classification of the allergic rhinitis have been significantly changed in 2001 and thereafter in 2003, based on recommendations of ARIA (Allergic Rhinitis and its Impact on Asthma) and in support of WHO (World Health Organization) and the disease is redefined as the most common chronic respiratory disease associated with asthma, being at the same time the major risk factor for its development [2]. Due to its chronic course and intermittent/persistent symptoms, it greatly affects quality of life. Due to the increasing incidence of AR, the recommendations related to application of antihistamines (loratadine, desloratadine) along with topical nasal corticosteroids (Mometasone furoate, Fluticasone propionate, Beclomethasone Dipropionate) [3] have been proposed based on the harmonized EAACI (European Academy of Allergology and Clinical Immunology) i ARIA recommendations. Based on ARIA recommendations, the intermittent rhinitis is defined as a disease associated with symptoms present <4 days per week or <4 weeks per year. Persistent rhinitis is defied as a disease characterized by presence of symptoms for more than 4 days a week and for more than 4 consecutive weeks per year. The symptoms include sneezing, nasal itching, nasal secretion, nasal obstructions and nasal congestion. Association between allergic rhinitis and other respiratory tract diseases, such as bronchial asthma, sinusitis, otitis media, nasalsinusal polyposis and respiratory infections is clinically evidenced, and thus comorbidity is increasingly frequently mentioned. Allergic rhinitis and asthma may currently be described as continuous inflammation, affecting the airways in different periods of life. The signs of rhinitis are found in 30-80% of patients affected with asthma while approximately 38-40% if the patients affected with allergic rhinitis will also develop asthma. Asthma develops in patients with allergic rhinitis three times as frequent as in rhinitis-free individuals [7,8,9]. Approximately 20 % of children with AR are predisposed to asthma, which appears more frequently in children with persistent rhinitis in comparison to those with allergic rhinitis. Both diseases appear concomitantly in 25% of the patients. Polyposis of the nasal sinuses develops more frequently in nonatopic rhinitis and asthma, particularly in individuals suffering from non-allergic hypersensitive aspirin-induced reaction, the so cal-led aspirin triad. Persistent rhinitis caused by mites, due to the protracted, more intensive inflammation, more frequently results in polyposis when compared to allergic rhinitis caused by pollens. In addition to synthesis of high concentrations of the total and specific IgE, the individuals with atopic constitution also have lower IgA concentrations, which makes favorable conditions for onset of different infections due to liability of the mucosal membrane [9,10]. The advantage of the last generation of anti-histamines lies in the fact that in addition to anti-allergic effects (mast cells and their mediators) they also have significant antiinflammatory effects (eosinophils and their mediators), as well as antiviral effects (desloratadine prevents actions of the ICAM-1 - intercellular adhesion molecule, which is a receptor for approximately 90% of rhino-viruses). Thus, administration of antihistamines in treatment of viral infections in patients with allergic rhinitis is justifiable. Administration of desloratadine as a potent, selective H1 receptor blocker free of sedative effects, cognitive or psychomotor disorders or anticholinergic effects which is safe even in children aged ≥2 is recommended. Mometasone furoate is a highly potent intranasal spray, highly effective in prophylaxis and treatment of the seasonal allergic rhinitis (SAR) as well as in treatment of perennial allergic rhinitis (PAR) in both adult and pediatric patients. It may be applied in treatment of SAR and PAR in adults and children aged ≥ 2 years, as well as in prophylaxis of SAR in patients aged ≥ 12 years. Owing to its low systemic actions, it has no hypothalamic pituitary adrenal axis (HPA) effects or the effects on bone growth in prepubertal children [5,11]. It may be applied as a monotherapy or in combination with antihistamines or other corticosteroids, depending on clinical picture of the disease [11,12]. Allergen-specific immunotherapy is an efficient etiological therapy in the case of allergic respiratory diseases caused by the IgE mechanism. AIM OF WORK The paper was aimed at presenting efficacy of administration of Mometasone furoate in the form of intranasal spray in treatment and/or prophylaxis of intermittent or persistent form of the allergic rhinitis. METHOD Allergic rhinitis diagnosis is based on detailed and targeted personal and family history of the symptomatic patients, clinical ENT examination (anterior and posterior rhinoscopy) indirect epipharygnoscopy (presence of enlarged adenoid vegeta-tion that might influence clinical picture – nasal obstruction), bacterial isolate findings (in case of positive findings, complete eradication would be necessary) and allergy tests, including skin prick test to standard inhalation allergens (Pastorel-lo,1994). Essentially, the reaction results from the contact between allergens and mast cells of the skin, which have on their surfaces specific IgE antibodies, and in case of presence of IgE antibody to the specific allergen, the mediators are released from the mast cells resulting in development of the skin wheal (within 12-15 minutes subsequent to the contact with the allergen). RAST test (evidences presence of IgE antibodies specific for certain allergen) and RIST test (radioimmunosorbent test, useful for quantitative determination of IgE immunoglobulins), make allergologic evaluation complete, although the incidence of application of the tests is increasingly lower. FESS (functional endoscopic sinus surgery) as a sophisticated method for visualization of the nasal sinuses is applied as diagnostic and therapeutic method. In an effort for find out as safe as possible medicine from the GCS group (glucocorticosteroid) having in mind suppression of growth and osteoporosis, particularly in childhood, short half-life and duration and method of drug administration, were the crucial factors for finding the medicine with good topical effects and minimal systemic effects . Highly potent non-fluorinated corticosteroid – Mometasone furoate free of any suppressive effects on the hypothalamic pituitary adrenal axis. With rapid onset of action (within 5/7 h after its ad-ministration) and low bioavailability, Mometasone furoate is classified into the group of drugs with supreme systemic safety, excellent and documented efficacy (complete or significantly reduction of symptoms as early as within 72 h in 70% of the cases), and possible administration as early as from the age of 2, with exceptionally good local tolerance [11,12]. Single drug dose contains 0.05µg Mometasone furoate, cellulose BP 65 cps, glycerol, sodium di-hydrate citrate, citric acid monohydrate, polysorbate 80, benzalkonium chloride, phenylethyl alcohol and purified water. The drug was applied as a single dose, in the morning in children aged ≥ 2 - 12.years, one dose once a day (50µg/dose, i.e., 1 dose containing 50 mcg Mometasone furoate) in each nostril; in children above 12 years of age, two doses were applied in each nostril once a day. Optimal daily dose is 100µg, as a single dose in children aged ≥2 up to 12 years of age and 200µg in children aged ≥12 and adults. RESULTS Mometasone furoate nasal spray was applied as monotherapy in 267 patients aged 2-18 years in 2007, out of whom 89.51% were school children. The total of 164 patients were males (61.42%). All the patients were subjected to prick test and positive responses were obtained in 257 patients (96.25%), while in 10 patients, negative response was not correlated to the clinical findings, and therefore they underwent medicament treatment under the diagnosis of allergic rhinitis. The studies evidenced that the majority of the patients have had positive findings to grass pollens, house mites, weed pollens, house dust and tree pollens while positive findings to molds, tobacco and animal hair were significantly less frequent The total of 37 children, with their age ranging between 1 and 5 years were adenoidectomized based on indications. Seasonal and perennial rhinitis were diagnosed in 91 (34.08%) and 176 patients (65.92%), respecttively (Graph 1). Grass pollen (the most significant due to their crossreactivity) and weed pollen were dominant allergens in seasonal rhinitis, while mites (Dermatophagoides pteronyssinus) were predominant in the persistent rhinitis. 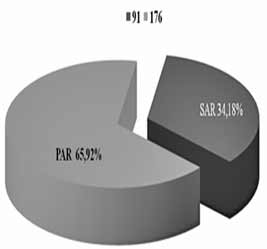 Graph 1. Distribution of patients according to allergic rhinitis type The leading symptoms included the following: sneezing (outbursts in the morning upon awakening), accompanied by nasal and eye itching, nasal secretion, nasal obstruction and nasal congestions. Clinical drug effects were monitored based on reduction of clinical symptoms and mucosal membrane findings. Owing to rapid onset of drug action ensuing within 5-7 h, significant reduction of symptoms and signs was observed in one third of the patients, while after 72 h, symptom withdrawal was significant in 167 patients (62.54%). After 2 weeks of drug administration, positive response was achieved in more than 70% of the patients (Graph 2). Sneezing, nasal secretion and nasal itching were reduced in 61 patients (67.03%) with seasonal rhinitis (Graph 3). Nasal congestion, nasal obstruction and nasal secretion subsided on 132 patients (75.56%) with perennial rhinitis. (Graph 4) Cough as a frequent AR symptom, particularly in SAR caused by postnasal drip (secretion), was discontinued after 2 weeks of drug administration in high percentage of patients. Nocturnal nasal congestion, nasal itching and sneezing were significantly reduced. 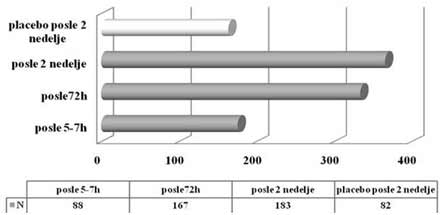 Graph 2. Distribution of patients according to MF efficacy 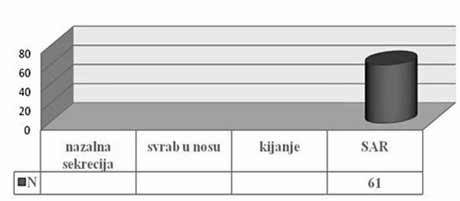 Graph 3. Distribution of patients according to withdrawal of symptoms 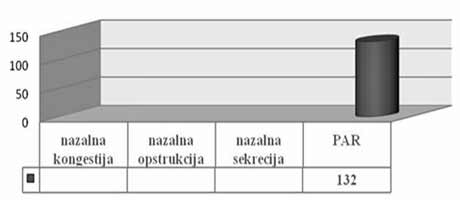 Graph 4. Distribution of patients according to withdrawal of symptoms |
||||||||
| Drug effect was monitored
according to the clinical picture and presence of symptoms in the group
in which the drug was applied as well as in the same number of control
subjects who received placebo. The drug was applied for 2/4/6 months and
up to one year, either continuously or intermittently ac-cording to
indications. Method and duration of drug administration were adjusted to the type of allergic rhinitis, patient’s age, clinical picture and degree of symptoms. The mechanism of drug action may be explained by inhibition of synthesis and/or release of inflammation mediators, early and late phase (lymphocytes, mast cells, basophils and eosinophils). Nasal mucosa of the patients with allergic rhinitis exhibits the epithelium with focal squamous metaplasia and intraepithelial inflammatory infiltrate with large number of eosinophils in the lamina propria. After drug application, squamous metaplasia or intraepithelial inflammatory infiltration and subepithelial eosinophilic inflammation were not observed on the mucosal bioptic material due to the reduction of changes. No signs of mucosal atrophy were evidenced. Within prophylaxis of pollen-induced rhinitis, the treatment starts 3-4 weeks before the season. DISCUSSIONIn addition to asthma, allergic rhinitis is the second most frequent
chronic respiratory disease. As a perennial or seasonal due to its long
presence of symptoms, it has major influence on quality of life
regardless of the patients’ age, particularly in children. Due to lack
of sleep and persistent symptoms (sneezing, nasal and eye icthing, nasal
congestion and obstruction both nocturnal and diurnal) it prevents
everyday activities due to lower level of concentration or its complete
loss in the school and during sports, which is reflected in both psychic
and emotional development of a child. CONCLUSIONAllergic rhinitis, as a local manifestation of the systemic allergic
reaction is treated with Mometasone furoate owing to its superior
systemic safety, high efficacy and exceptional local tolerance. The drug
is applied both prophylactically and therapeutically in cases with
seasonal allergic rhinitis as well as in treatment of persistent
allergic rhinitis of different etiology in patients aged 2 – 18 years as
a single drug. REFERENCES
|
||||||||
|
Corresponding Address: Vesna Stojanović Kamberović Dom zdravlja”Zvezdara”,Beograd Olge Jovanović br.11.Beograd mob.tel 063/ 77 98 390 e mail: vesnanbgd@yahoo.com Paper received: 23.7.2008 Paper accepted: 30.9.2008 Published online: 20.10.2008 |
||||||||
|
|
||||||||
| Infotrend Crea(c)tive Design | ||||||||
